Subretinal AAV delivery of RNAi-therapeutics targeting VEGFA reduces choroidal neovascularization in a large animal model
- PMID: 38605811
- PMCID: PMC11007540
- DOI: 10.1016/j.omtm.2024.101242
Subretinal AAV delivery of RNAi-therapeutics targeting VEGFA reduces choroidal neovascularization in a large animal model
Abstract
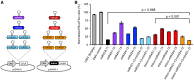
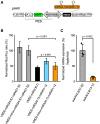
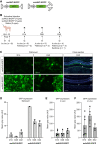
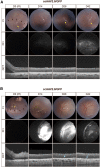
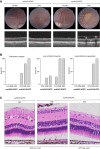
Neovascular age-related macular degeneration (nAMD) is a frequent cause of vision loss among the elderly in the Western world. Current disease management with repeated injections of anti-VEGF agents accumulates the risk for adverse events and constitutes a burden for society and the individual patient. Sustained suppression of VEGF using gene therapy is an attractive alternative, which we explored using adeno-associated virus (AAV)-based delivery of novel RNA interference (RNAi) effectors in a porcine model of choroidal neovascularization (CNV). The potency of VEGFA-targeting, Ago2-dependent short hairpin RNAs placed in pri-microRNA scaffolds (miR-agshRNA) was established in vitro and in vivo in mice. Subsequently, AAV serotype 8 (AAV2.8) vectors encoding VEGFA-targeting or irrelevant miR-agshRNAs under the control of a tissue-specific promotor were delivered to the porcine retina via subretinal injection before CNV induction by laser. Notably, VEGFA-targeting miR-agshRNAs resulted in a significant and sizable reduction of CNV compared with the non-targeting control. We also demonstrated that single-stranded and self-complementary AAV2.8 vectors efficiently transduce porcine retinal pigment epithelium cells but differ in their transduction characteristics and retinal safety. Collectively, our data demonstrated a robust anti-angiogenic effect of VEGFA-targeting miR-aghsRNAs in a large translational animal model, thereby suggesting AAV-based delivery of anti-VEGFA RNAi therapeutics as a valuable tool for the management of nAMD.
Keywords: AAV; AMD; CNV; RNAi therapeutics; anti-VEGF; large animal model; miR-agshRNA; pig; retinal gene therapy.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Rupert R.A.B., Jost B.J., Alain M.B., Maria Vittoria C., Aditi D., Seth R.F., David S.F., Jill E.K., John H.K., Janet L., et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. Br. J. Ophthalmol. 2018;102:575–585. doi: 10.1136/bjophthalmol-2017-311258. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

