Development of adenoviral vectors that transduce Purkinje cells and other cerebellar cell-types in the cerebellum of a humanized mouse model
- PMID: 38605812
- PMCID: PMC11007541
- DOI: 10.1016/j.omtm.2024.101243
Development of adenoviral vectors that transduce Purkinje cells and other cerebellar cell-types in the cerebellum of a humanized mouse model
Abstract
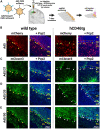
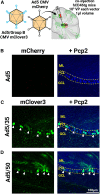
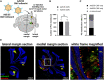
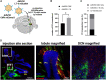
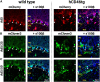
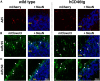
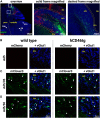
Viral vector gene therapy has immense promise for treating central nervous system (CNS) disorders. Although adeno-associated virus vectors (AAVs) have had success, their small packaging capacity limits their utility to treat the root cause of many CNS disorders. Adenoviral vectors (Ad) have tremendous potential for CNS gene therapy approaches. Currently, the most common vectors utilize the Group C Ad5 serotype capsid proteins, which rely on the Coxsackievirus-Adenovirus receptor (CAR) to infect cells. However, these Ad5 vectors are unable to transduce many neuronal cell types that are dysfunctional in many CNS disorders. The human CD46 (hCD46) receptor is widely expressed throughout the human CNS and is the primary attachment receptor for many Ad serotypes. Therefore, to overcome the current limitations of Ad vectors to treat CNS disorders, we created chimeric first generation Ad vectors that utilize the hCD46 receptor. Using a "humanized" hCD46 mouse model, we demonstrate these Ad vectors transduce cerebellar cell types, including Purkinje cells, that are refractory to Ad5 transduction. Since Ad vector transduction properties are dependent on their capsid proteins, these chimeric first generation Ad vectors open new avenues for high-capacity helper-dependent adenovirus (HdAd) gene therapy approaches for cerebellar disorders and multiple neurological disorders.
Keywords: CD46; Purkinje cell; cerebellum; chimeric adenoviral vector; helper-dependent adenoviral vector; retrograde transduction; tropism; viral vector gene therapy.
© 2024 The Author(s).
Conflict of interest statement
E.K. and S.M.Y. have a provisional patent filed related to the findings in the manuscript.
Figures

Similar articles
-
Development and evaluation of helper dependent adenoviral vectors for inner ear gene delivery.Hear Res. 2023 Aug;435:108819. doi: 10.1016/j.heares.2023.108819. Epub 2023 May 26. Hear Res. 2023. PMID: 37276687 Free PMC article.
-
Transduction of brain dopamine neurons by adenoviral vectors is modulated by CAR expression: rationale for tropism modified vectors in PD gene therapy.PLoS One. 2010 Sep 17;5(9):e12672. doi: 10.1371/journal.pone.0012672. PLoS One. 2010. PMID: 20862245 Free PMC article.
-
Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration.Gene Ther. 1999 Sep;6(9):1565-73. doi: 10.1038/sj.gt.3300995. Gene Ther. 1999. PMID: 10490766
-
Adenovirus vectors composed of subgroup B adenoviruses.Curr Gene Ther. 2007 Aug;7(4):229-38. doi: 10.2174/156652307781369137. Curr Gene Ther. 2007. PMID: 17969556 Review.
-
[Characterization of adenovirus serotype 35 vectors using genetically modified animals and non-human primates].Yakugaku Zasshi. 2006 Nov;126(11):1013-9. doi: 10.1248/yakushi.126.1013. Yakugaku Zasshi. 2006. PMID: 17077607 Review. Japanese.
References
Grants and funding
LinkOut - more resources
Full Text Sources

