Targeted therapeutic options in early and metastatic NSCLC-overview
- PMID: 38605928
- PMCID: PMC11006988
- DOI: 10.3389/pore.2024.1611715
Targeted therapeutic options in early and metastatic NSCLC-overview
Abstract
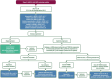
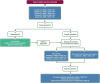
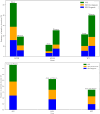
The complex therapeutic strategy of non-small cell lung cancer (NSCLC) has changed significantly in recent years. Disease-free survival increased significantly with immunotherapy and chemotherapy registered in perioperative treatments, as well as adjuvant registered immunotherapy and targeted therapy (osimertinib) in case of EGFR mutation. In oncogenic-addictive metastatic NSCLC, primarily in adenocarcinoma, the range of targeted therapies is expanding, with which the expected overall survival increases significantly, measured in years. By 2021, the FDA and EMA have approved targeted agents to inhibit EGFR activating mutations, T790 M resistance mutation, BRAF V600E mutation, ALK, ROS1, NTRK and RET fusion. In 2022, the range of authorized target therapies was expanded. With therapies that inhibit KRASG12C, EGFR exon 20, HER2 and MET. Until now, there was no registered targeted therapy for the KRAS mutations, which affect 30% of adenocarcinomas. Thus, the greatest expectation surrounded the inhibition of the KRAS G12C mutation, which occurs in ∼15% of NSCLC, mainly in smokers and is characterized by a poor prognosis. Sotorasib and adagrasib are approved as second-line agents after at least one prior course of chemotherapy and/or immunotherapy. Adagrasib in first-line combination with pembrolizumab immunotherapy proved more beneficial, especially in patients with high expression of PD-L1. In EGFR exon 20 insertion mutation of lung adenocarcinoma, amivantanab was registered for progression after platinum-based chemotherapy. Lung adenocarcinoma carries an EGFR exon 20, HER2 insertion mutation in 2%, for which the first targeted therapy is trastuzumab deruxtecan, in patients already treated with platinum-based chemotherapy. Two orally administered selective c-MET inhibitors, capmatinib and tepotinib, were also approved after chemotherapy in adenocarcinoma carrying MET exon 14 skipping mutations of about 3%. Incorporating reflex testing with next-generation sequencing (NGS) expands personalized therapies by identifying guideline-recommended molecular alterations.
Keywords: NSCLC; central nervous system efficacy; driver oncogenes; molecular testing; targeted therapy.
Copyright © 2024 Gálffy, Morócz, Korompay, Hécz, Bujdosó, Puskás, Lovas, Gáspár, Yahya, Király and Lohinai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- NCCN. NCCN guidelines non-small cell lung cancer version 1 (2024). Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed January 30, 2024).
-
- Ahn M, Arcila M, Bazhenova L, Beasley M, Berezowska S, Bubendorf L, et al. IASLC atlas of molecular testing for targeted therapy in lung cancer. Denver: International Association for the Study of Lung Cancer; (2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

