MiR-144-5p and miR-21-5p do not drive bone disease in a mouse model of type 1 diabetes mellitus
- PMID: 38606150
- PMCID: PMC11008730
- DOI: 10.1093/jbmrpl/ziae036
MiR-144-5p and miR-21-5p do not drive bone disease in a mouse model of type 1 diabetes mellitus
Abstract
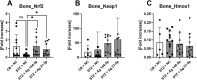
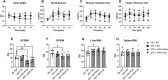
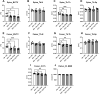
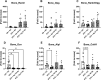
The increased risk of fractures in patients with type 1 diabetes mellitus (T1DM) is nowadays well recognized. However, the exact mechanism of action of diabetic bone disease has not been fully elucidated. MicroRNAs (miRNAs) are gene regulators that operate post-transcriptionally and have been implicated in the development of various metabolic disorders including T1DM. Previous studies have implicated a role for miR-144-5p and miR-21-5p, which are involved in controlling oxidative stress by targeting Nrf2, in T1DM. To date, it is unclear whether miR-144-5p and miR-21-5p affect bone health in T1DM. Thus, this study aimed to investigate the influence of miR-144-5p and miR-21-5p knockdown in the development of bone disease in T1DM male mice. Therefore, T1DM was induced in 10-wk-old male mice using streptozotocin (STZ). One week later, after development of hyperglycemia, antagomir-144-5p and antagomir-21-5p or their non-targeting control were administered at 10 mg/kg BW once a week until the end of the experiment. At 14 wk of age, glucose levels, bone, and fat mass were analyzed. The results revealed that treating T1DM male mice with antagomir-144-5p and antagomir-21-5p did not protect against diabetes development or bone loss, despite the successful downregulation of the miRNAs and the normalization of Nrf2 mRNA levels in bone tissue. Histological and serological parameters of bone formation or resorption were not altered by the antagomir treatment. Finally, we measured the expression of miRNA-144-5p or miRNA-21-5p in the serum of 30 individuals with T1DM and compared them to non-diabetic controls, but did not find an altered expression of either miRNA. In conclusion, the knockdown of miR-144-5p and miR-21-5p does not affect STZ-induced diabetes development or loss of bone mass in male mice. However, it does normalize expression of the anti-oxidant factor Nrf2 in diabetic bone tissue.
Keywords: T1DM; antagomir; bone loss; miR-144-5p; miR-21-5p.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
M.B. received honoraria as a consultant and speaker from Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Lilly, Novo Nordisk, Novartis, and Sanofi. L.C.H. received honoraria as a consultant and speaker from Amgen, Novo Nordisk, and UCB and support for clinical trials from Ascendis. M.R. received honoraria as a speaker from UCB and Santhera. M.H. is CEO and cofounder of TAmiRNA GmbH. M.H. and D.C.V. are employees of TAmiRNA GmbH. S.D. has no conflict of interest.
Figures





Similar articles
-
MicroRNA expression profiles and type 1 diabetes mellitus: systematic review and bioinformatic analysis.Endocr Connect. 2017 Nov;6(8):773-790. doi: 10.1530/EC-17-0248. Epub 2017 Oct 6. Endocr Connect. 2017. PMID: 28986402 Free PMC article.
-
Altered Expression of Vitamin D Metabolism Genes and Circulating MicroRNAs in PBMCs of Patients with Type 1 Diabetes: Their Association with Vitamin D Status and Ongoing Islet Autoimmunity.Noncoding RNA. 2023 Oct 7;9(5):60. doi: 10.3390/ncrna9050060. Noncoding RNA. 2023. PMID: 37888206 Free PMC article.
-
Histone Deacetylase 3 Aggravates Type 1 Diabetes Mellitus by Inhibiting Lymphocyte Apoptosis Through the microRNA-296-5p/Bcl-xl Axis.Front Genet. 2020 Nov 2;11:536854. doi: 10.3389/fgene.2020.536854. eCollection 2020. Front Genet. 2020. PMID: 33240312 Free PMC article.
-
Circulating serum microRNAs including senescent miR-31-5p are associated with incident fragility fractures in older postmenopausal women with type 2 diabetes mellitus.Bone. 2022 May;158:116308. doi: 10.1016/j.bone.2021.116308. Epub 2022 Jan 21. Bone. 2022. PMID: 35066213
-
Network pharmacology-based identification of miRNA expression of Astragalus membranaceus in the treatment of diabetic nephropathy.Medicine (Baltimore). 2022 Feb 4;101(5):e28747. doi: 10.1097/MD.0000000000028747. Medicine (Baltimore). 2022. PMID: 35119030 Free PMC article.
Cited by
-
microRNAs in Type 1 Diabetes: Roles, Pathological Mechanisms, and Therapeutic Potential.Int J Mol Sci. 2025 Apr 2;26(7):3301. doi: 10.3390/ijms26073301. Int J Mol Sci. 2025. PMID: 40244147 Free PMC article. Review.
-
Exploratory miRNA profiling from serum and bone tissue of mice with T1D-induced bone loss.Front Endocrinol (Lausanne). 2024 Dec 24;15:1477257. doi: 10.3389/fendo.2024.1477257. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39777220 Free PMC article.
References
LinkOut - more resources
Full Text Sources

