Optimizing fluconazole-embedded transfersomal gel for enhanced antifungal activity and compatibility studies
- PMID: 38606182
- PMCID: PMC11007155
- DOI: 10.3389/fphar.2024.1353791
Optimizing fluconazole-embedded transfersomal gel for enhanced antifungal activity and compatibility studies
Abstract
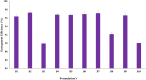
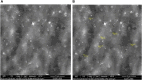
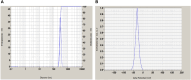
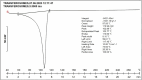
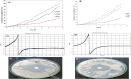
Fungal infections are of major concern all over the globe, and fluconazole is the most prevalently used drug to treat it. The goal of this research work was to formulate a fluconazole-embedded transfersomal gel for the treatment of fungal infections. A compatibility study between fluconazole and soya lecithin was performed by differential scanning calorimetry (DSC). Transfersomes were formulated by a thin-film hydration technique using soya lecithin and Span 80. A central composite design was adopted to prepare different formulations. Soya lecithin and Span 80 were chosen as independent variables, and the effect of these variables was studied on in vitro drug diffusion. Formulations were evaluated for entrapment efficiency and in vitro drug diffusion. The results of in vitro drug diffusion were analyzed using the analysis of variance (ANOVA) test. Optimized formulation was prepared based on the overlay plot and evaluated by scanning electron microscopy, DSC, vesicle size, polydispersity index (PDI), zeta potential, and in vitro drug diffusion studies. An optimized formulation was loaded into xanthan gum gel base and evaluated for pH, viscosity, in vitro and ex vivo drug diffusion, and antifungal activity. DSC studies revealed compatibility between fluconazole and soya lecithin. Entrapment efficiency and in vitro drug diffusion of various formulations ranged between 89.92% ± 0.20% to 97.28% ± 0.42% and 64% ± 1.56% to 85% ± 2.05%, respectively. A positive correlation was observed between in vitro drug diffusion and Span 80; conversely, a negative correlation was noted with soya lecithin. Entrapment efficiency, particle size, zeta potential, PDI, and drug diffusion of optimized formulation were 95.0% ± 2.2%, 397 ± 2 nm, -38 ± 5 mV, 0.43%, and 81 % ± 2%, respectively. SEM images showed well-distributed spherical-shaped transfersomes. In vitro, ex vivo drug diffusion and antifungal studies were conclusive of better diffusion and enhanced antifungal potential fluconazole in transfersomal formulation.
Keywords: ex-vivo studies; fluconazole; topical application; transfersomal gel; transfersomes.
Copyright © 2024 Cheng, Kandekar, Ma, Bhabad, Pandit, Liu, Luo, Munot, Chorage, Patil, Patil and Tao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Balata G. F., Faisal M. M., Elghamry H. A., Sabry S. A. (2020). Preparation and characterization of Ivabradine HCl transfersomes for enhanced transdermal delivery. J. Drug Deliv. Sci. Technol. 60, 101921. 10.1016/j.jddst.2020.101921 - DOI
-
- Barani M., Bilal M., Rahdar A., Arshad R., Kumar A., Hamishekar H., et al. (2021). Nanodiagnosis and nanotreatment of colorectal cancer: an overview. J. Nanopart. Res. 23 (1), 18–25. 10.1007/s11051-020-05129-6 - DOI
-
- Bhasin B., Londhe V. Y. (2018). An overview of transfersomal drug delivery. Int. J. Pharm. Sci. Res. 9 (6), 2175–2184. 10.13040/IJPSR.0975-8232.9(6).2175-84 - DOI
-
- Bousmina M. (1999). Rheology of polymer blends: linear model for viscoelastic emulsions. Rheol. Acta 38 (1), 73–83. 10.1007/s003970050157 - DOI
LinkOut - more resources
Full Text Sources

