MOLECULAR DETECTION PROTOCOL OF SARS-COV-2 THROUGH SELF-COLLECTED SALIVA SPECIMENS VERSUS NASOPHARYNGEAL SWABS
- PMID: 38606190
- PMCID: PMC11004780
- DOI: 10.21010/Ajidv18i2.1
MOLECULAR DETECTION PROTOCOL OF SARS-COV-2 THROUGH SELF-COLLECTED SALIVA SPECIMENS VERSUS NASOPHARYNGEAL SWABS
Abstract
Background: Several reports have shown that saliva specimen is an excellent alternative biofluid sample for SARS-CoV-2 detection. We conducted this study, in order to assess the sensitivity and specificity of using saliva self-collected by adult and pediatric patients, as a biological sample for RT-PCR diagnosis.
Aims: The present study was carried out to assess the sensitivity and specificity of using saliva self-collected from adult and pediatric patients, as a biological sample for RT-qPCR diagnosis.
Methods: In this study, 50 symptomatic patients and 40 asymptomatic subjects (adult and pediatric) were enrolled between September 2020 and November 2020 at the Department of Infectious Diseases, Bejaia University Hospital (CHU), and tested simultaneously for the sensitivity and specificity of the SARS-CoV-2 viral genome by RT-PCR on both nasopharyngeal swabs NP swab and saliva samples.
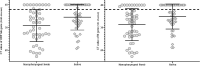
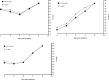
Results: Our RT-qPCR results revealed that saliva samples showed the highest sensitivity (95% CI [27.67, 29.82]) followed by a nasopharyngeal swab for symptomatic (95% CI [29.64, 31.49]) as well as for asymptomatic adult patients. Moreover, the saliva of symptomatic and asymptomatic patients was monitored, and the presence of viral RNA was detected in >95% of the asymptomatic patients as well as the symptomatic patients. Surprisingly, the Ct values of ORF1ab and N genes are highly lower in nasopharyngeal swabs compared to saliva. Indeed, the mean difference note that for the ORF1ab gene and N gene, the mean of difference in ΔCt value were respectively 3.683 and 3.578. Together, including symptomatic and asymptomatic subjects, the overall agreement between the saliva sample and the nasopharyngeal is about 84%.
Conclusion: The sensitivity of saliva samples remains acceptable; it may still be a viable option in locations where laboratory facilities are lacking for diagnostic purposes in the early phase of the disease.
Keywords: Diagnosis; Nasopharyngeal swab; Reverse Transcriptase Polymerase Chain Reaction; SARS-CoV-2; Saliva.
Copyright: © 2024 Afr. J. Infect. Diseases.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s). List of Abréviations:ACE2:Angiotensin-Converting Enzyme 2CoV:CoronavirusCt:Cycle thresholdNPS:nasopharyngeal swabOP: Oropharyngeal ORF:Open Reading Frames.RT-qPCR:real-time quantitative reverse transcriptase-polymerase chain reactionSARS-CoV-2:Severe Acute Respiratory Syndrome Coronavirus-2TMPRSS2:Transmembrane Serine Protease 2.
Figures

References
-
- Amirouche A, Ait-Ali D, Nouri H, Boudrahme-Hannou L, Tliba S, Ghidouche A, Bitam I. TRIzol-Based RNA Extraction for Detection Protocol for SARS-CoV-2 of Coronavirus Disease 2019. New Microbes and New Infections. 2021;41(mai):100874. https://doi.org/10.1016/j.nmni.2021.100874. - PMC - PubMed
-
- Báez-Santos, Yahira M, Sarah E, John St, Andrew D. Mesecar. The SARS-Coronavirus Papain-like Protease:Structure, Function and Inhibition by Designed Antiviral Compounds. Antiviral Research. 2015;115(mars):21–38. https://doi.org/10.1016/j.antiviral.2014.12.015. - PMC - PubMed
-
- Beyerstedt, Stephany Expedito Barbosa Casaro and Érika Bevilaqua Rangel. COVID-19:Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. European Journal of Clinical Microbiology &Infectious Diseases. 2021;40(5):905–19. https://doi.org/10.1007/s10096-020-04138-6. - PMC - PubMed
-
- Butler-Laporte, Guillaume, Alexander Lawandi, Ian Schiller, Mandy Yao, Nandini Dendukuri, Emily G, McDonald, Todd C Lee. Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2:A Systematic Review and Meta-Analysis. JAMA Internal Medicine. 2021;181(3):353. https://doi.org/10.1001/jamainternmed.2020.8876. - PMC - PubMed
-
- Fan, Guang, Xuan Qin, Daniel N, Streblow, Cristina Magallanes Hoyos, Donna E, Hansel Comparison of SARS-CoV-2 PCR-Based Detection Using Saliva or Nasopharyngeal Swab Specimens in Asymptomatic Populations. Microbiology Spectrum. 2021;9(1):e00062–21. https://doi.org/10.1128/Spectrum.00062-21. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
