Evaluation of partial volume correction and analysis of longitudinal [18F]GTP1 tau PET imaging in Alzheimer's disease using linear mixed-effects models
- PMID: 38606196
- PMCID: PMC11008283
- DOI: 10.3389/fnimg.2024.1355402
Evaluation of partial volume correction and analysis of longitudinal [18F]GTP1 tau PET imaging in Alzheimer's disease using linear mixed-effects models
Abstract
Purpose: We evaluated the impact of partial volume correction (PVC) methods on the quantification of longitudinal [18F]GTP1 tau positron-emission tomography (PET) in Alzheimer's disease and the suitability of describing the tau pathology burden temporal trajectories using linear mixed-effects models (LMEM).
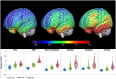
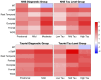
Methods: We applied van Cittert iterative deconvolution (VC), 2-compartment, and 3-compartment, and the geometric transfer matrix plus region-based voxelwise methods to data acquired in an Alzheimer's disease natural history study over 18 months at a single imaging site. We determined the optimal PVC method by comparing the standardized uptake value ratio change (%ΔSUVR) between diagnostic and tau burden-level groups and the longitudinal repeatability derived from the LMEM. The performance of LMEM analysis for calculating %ΔSUVR was evaluated in a natural history study and in a multisite clinical trial of semorinemab in prodromal to mild Alzheimer's disease by comparing results to traditional per-visit estimates.
Results: The VC, 2-compartment, and 3-compartment PVC methods had similar performance, whereas region-based voxelwise overcorrected regions with a higher tau burden. The lowest within-subject variability and acceptable group separation scores were observed without PVC. The LMEM-derived %ΔSUVR values were similar to the per-visit estimates with lower variability.
Conclusion: The results indicate that the tested PVC methods do not offer a clear advantage or improvement over non-PVC images for the quantification of longitudinal [18F]GTP1 PET data. LMEM offers a robust framework for the longitudinal tau PET quantification with low longitudinal test-retest variability.
Clinical trial registration: NCT02640092 and NCT03289143.
Keywords: Alzheimer's disease; linear mixed-effects models; longitudinal change; neuroimaging; tau PET.
Copyright © 2024 Sanabria Bohórquez, Baker, Manser, Tonietto, Galli, Wildsmith, Zou, Kerchner, Weimer and Teng.
Conflict of interest statement
SSB, PM, KW, RW, and ET are employees of Genentech, Inc. and/or shareholders in F. Hoffmann-La Roche, Ltd. MT, CG, YZ, and GK are employees of and shareholders in F. Hoffmann-La Roche, Ltd. SB is consultant of Genentech, Inc. The authors declare that this study received funding from Genentech, Inc. The funder had the following involvement in the study: Genentech was involved in the study design, data interpretation, and the decision to submit for publication in conjunction with the authors.
Figures



References
-
- Barthélemy N. R., Toth B., Manser P. T., Sanabria-Bohórquez S., Teng E., Keeley M., et al. . (2022). Site-specific cerebrospinal fluid tau hyperphosphorylation in response to Alzheimer's disease brain pathology: not all tau phospho-sites are hyperphosphorylated. J. Alzheimers Dis. 85, 415–429. 10.3233/JAD-210677 - DOI - PMC - PubMed

