Target-Mediated Fluoroquinolone Resistance in Neisseria gonorrhoeae: Actions of Ciprofloxacin against Gyrase and Topoisomerase IV
- PMID: 38606464
- PMCID: PMC11015056
- DOI: 10.1021/acsinfecdis.4c00041
Target-Mediated Fluoroquinolone Resistance in Neisseria gonorrhoeae: Actions of Ciprofloxacin against Gyrase and Topoisomerase IV
Abstract
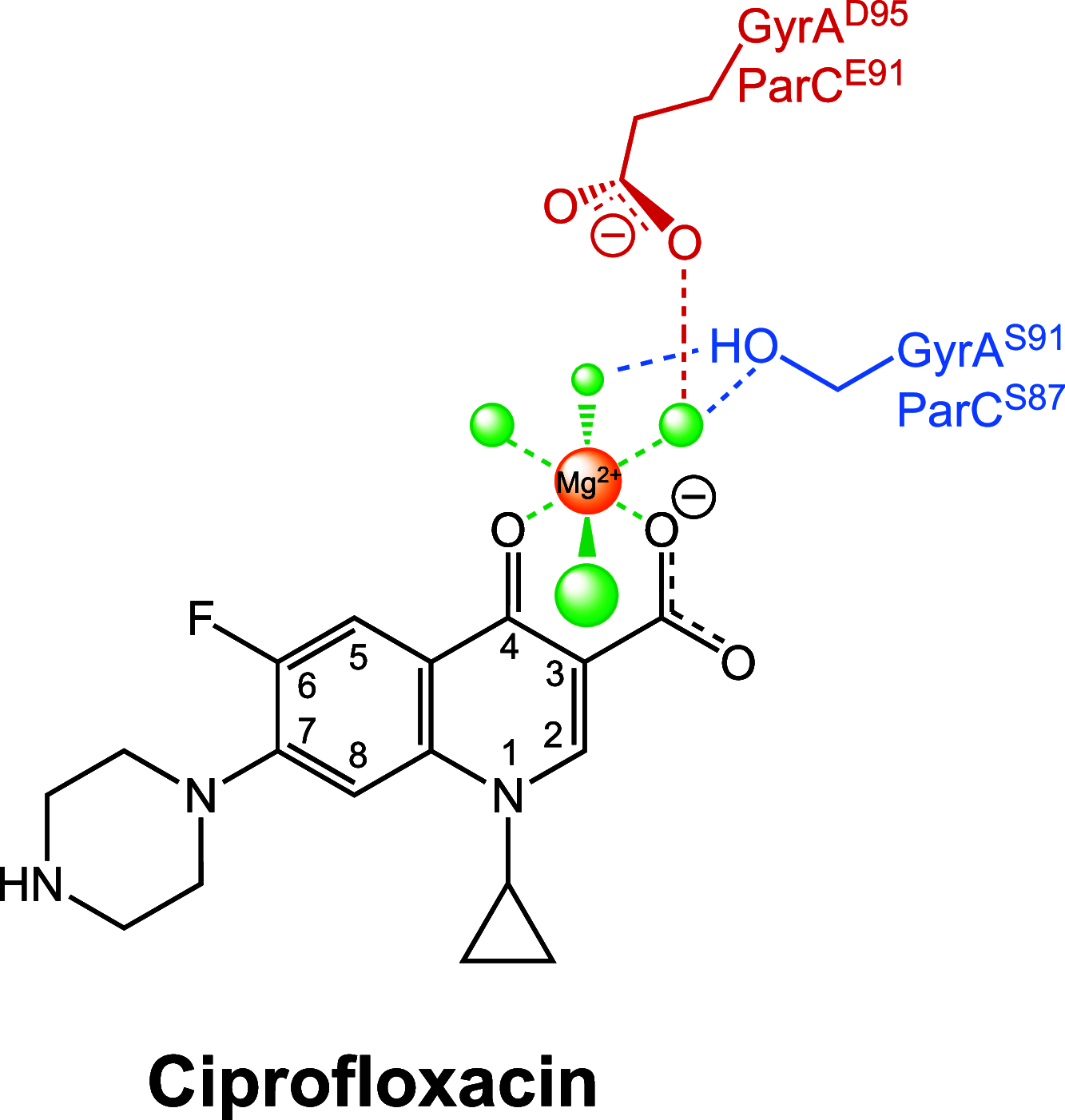
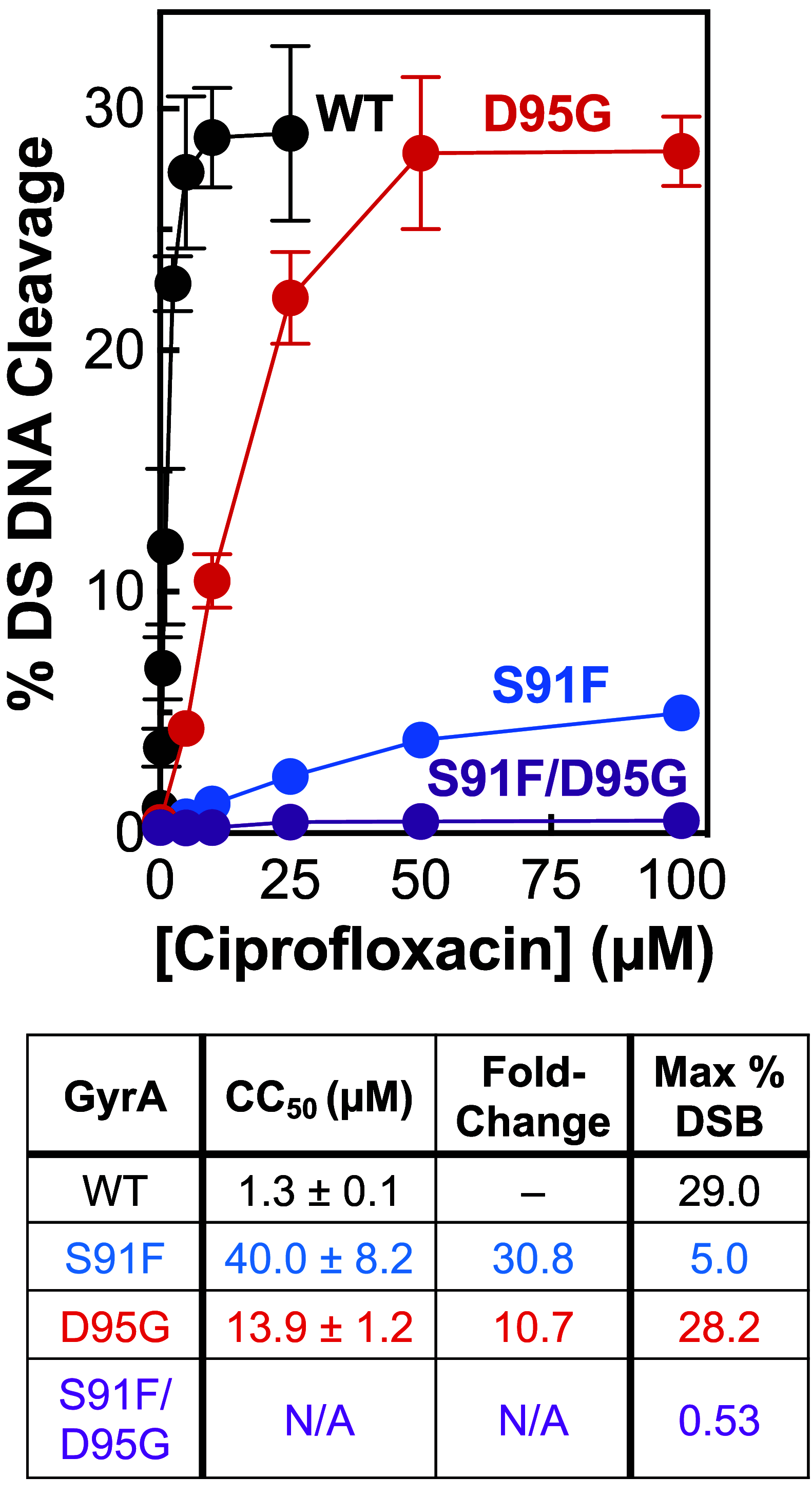
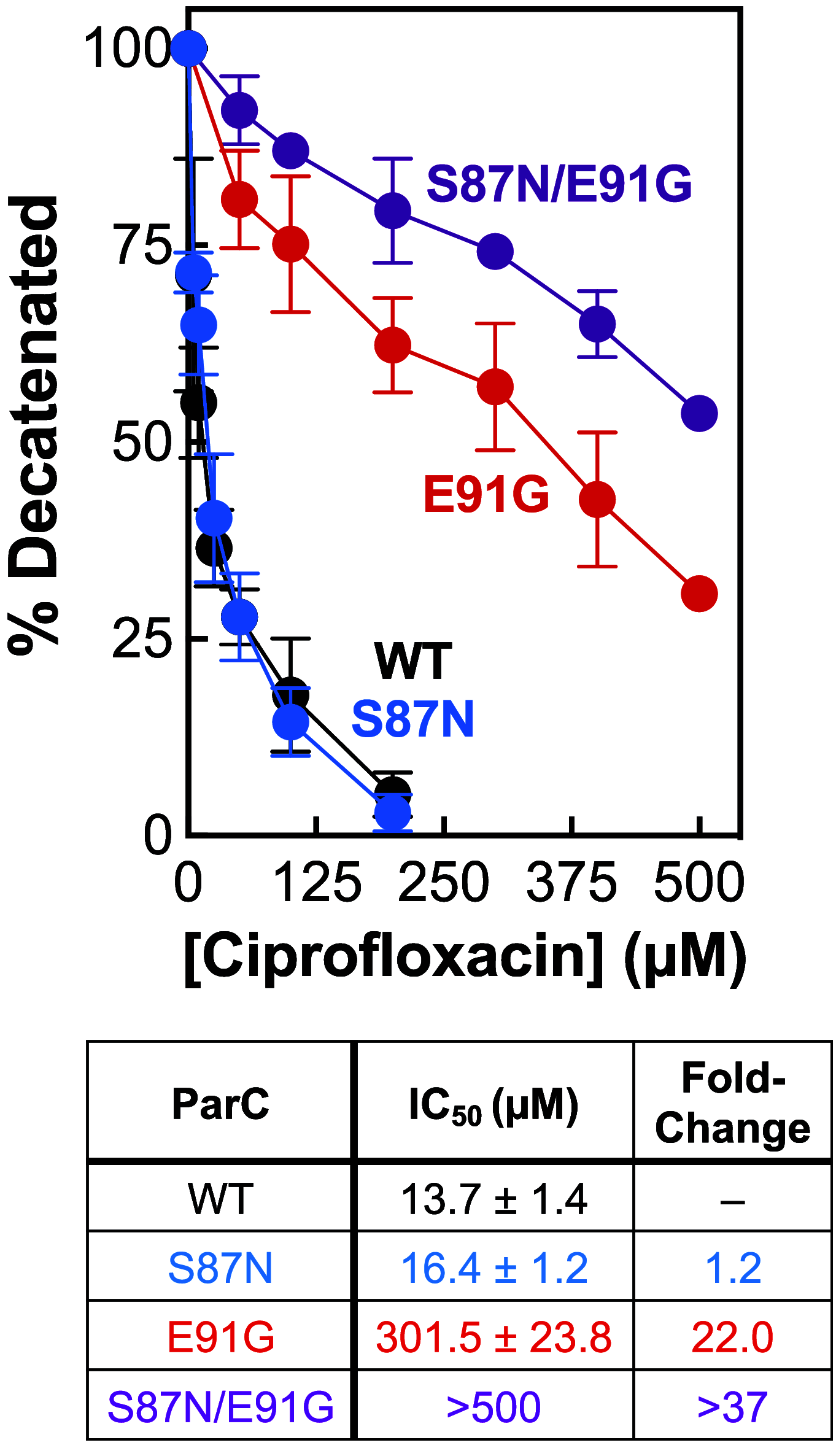
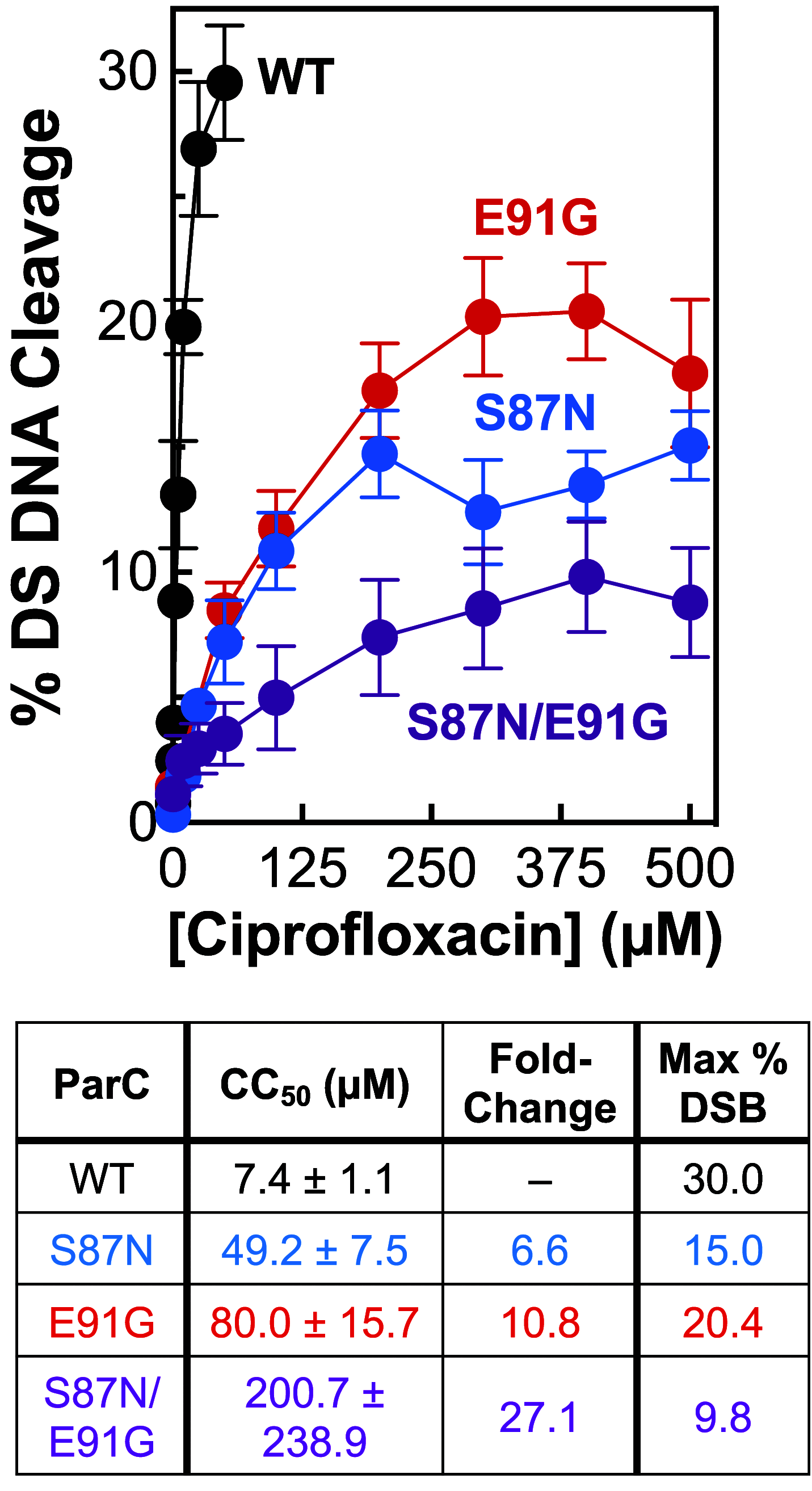
Fluoroquinolones make up a critically important class of antibacterials administered worldwide to treat human infections. However, their clinical utility has been curtailed by target-mediated resistance, which is caused by mutations in the fluoroquinolone targets, gyrase and topoisomerase IV. An important pathogen that has been affected by this resistance is Neisseria gonorrhoeae, the causative agent of gonorrhea. Over 82 million new cases of this sexually transmitted infection were reported globally in 2020. Despite the impact of fluoroquinolone resistance on gonorrhea treatment, little is known about the interactions of this drug class with its targets in this bacterium. Therefore, we investigated the effects of the fluoroquinolone ciprofloxacin on the catalytic and DNA cleavage activities of wild-type gyrase and topoisomerase IV and the corresponding enzymes that harbor mutations associated with cellular and clinical resistance to fluoroquinolones. Results indicate that ciprofloxacin interacts with both gyrase (its primary target) and topoisomerase IV (its secondary target) through a water-metal ion bridge that has been described in other species. Moreover, mutations in amino acid residues that anchor this bridge diminish the susceptibility of the enzymes for the drug, leading to fluoroquinolone resistance. Results further suggest that ciprofloxacin primarily induces its cytotoxic effects by enhancing gyrase-mediated DNA cleavage as opposed to inhibiting the DNA supercoiling activity of the enzyme. In conclusion, this work links the effects of ciprofloxacin on wild-type and resistant gyrase to results reported for cellular and clinical studies and provides a mechanistic explanation for the targeting and resistance of fluoroquinolones in N. gonorrhoeae.
Keywords: DNA cleavage; DNA supercoiling/decatenation; ciprofloxacin; fluoroquinolone; gyrase; topoisomerase IV.
Conflict of interest statement
The authors declare the following competing financial interest(s): PFC is a GSK employee and shareholder.
Figures

Similar articles
-
Gyrase and Topoisomerase IV: Recycling Old Targets for New Antibacterials to Combat Fluoroquinolone Resistance.ACS Infect Dis. 2024 Apr 12;10(4):1097-1115. doi: 10.1021/acsinfecdis.4c00128. Epub 2024 Apr 2. ACS Infect Dis. 2024. PMID: 38564341 Free PMC article. Review.
-
Interactions between Zoliflodacin and Neisseria gonorrhoeae Gyrase and Topoisomerase IV: Enzymological Basis for Cellular Targeting.ACS Infect Dis. 2024 Aug 9;10(8):3071-3082. doi: 10.1021/acsinfecdis.4c00438. Epub 2024 Jul 31. ACS Infect Dis. 2024. PMID: 39082980 Free PMC article.
-
In Vitro Activity of Sitafloxacin and Additional Newer Generation Fluoroquinolones Against Ciprofloxacin-Resistant Neisseria gonorrhoeae Isolates.Microb Drug Resist. 2018 Jan/Feb;24(1):30-34. doi: 10.1089/mdr.2017.0054. Epub 2017 Jun 5. Microb Drug Resist. 2018. PMID: 28581359
-
Inhibition of Neisseria gonorrhoeae Type II Topoisomerases by the Novel Spiropyrimidinetrione AZD0914.J Biol Chem. 2015 Aug 21;290(34):20984-20994. doi: 10.1074/jbc.M115.663534. Epub 2015 Jul 6. J Biol Chem. 2015. PMID: 26149691 Free PMC article.
-
Management of Neisseria gonorrhoeae infection: from drug resistance to drug repurposing.Expert Opin Ther Pat. 2024 Jun;34(6):511-524. doi: 10.1080/13543776.2024.2367005. Epub 2024 Jun 14. Expert Opin Ther Pat. 2024. PMID: 38856987 Review.
Cited by
-
Fluoroquinolones and Biofilm: A Narrative Review.Pharmaceuticals (Basel). 2024 Dec 11;17(12):1673. doi: 10.3390/ph17121673. Pharmaceuticals (Basel). 2024. PMID: 39770514 Free PMC article. Review.
-
PARP1-driven repair of topoisomerase IIIα DNA-protein crosslinks by FEN1.Cell Rep. 2024 Aug 27;43(8):114522. doi: 10.1016/j.celrep.2024.114522. Epub 2024 Jul 18. Cell Rep. 2024. PMID: 39028621 Free PMC article.
-
In vitro evolution of ciprofloxacin resistance in Neisseria commensals and derived mutation population dynamics in natural Neisseria populations.FEMS Microbiol Lett. 2025 Jan 10;372:fnae107. doi: 10.1093/femsle/fnae107. FEMS Microbiol Lett. 2025. PMID: 39788725 Free PMC article.
-
Gyrase and Topoisomerase IV: Recycling Old Targets for New Antibacterials to Combat Fluoroquinolone Resistance.ACS Infect Dis. 2024 Apr 12;10(4):1097-1115. doi: 10.1021/acsinfecdis.4c00128. Epub 2024 Apr 2. ACS Infect Dis. 2024. PMID: 38564341 Free PMC article. Review.
-
Assessing the Antibiotic Resistance in Food Lactic Acid Bacteria: Risks in the Era of Widespread Probiotic Use.Food Sci Nutr. 2025 Jul 31;13(8):e70740. doi: 10.1002/fsn3.70740. eCollection 2025 Aug. Food Sci Nutr. 2025. PMID: 40755505 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention . Outpatient antibiotic prescriptions—United States, 2017; U.S. Department of Health and Human Services, 2019. https://www.cdc.gov/antibiotic-use/data/report-2017.html (accessed Nov 12, 2023).
-
- Basarab G. S.Four ways to skin a cat: inhibition of bacterial topoisomerases leading to the clinic. In Antibacterials; Fisher J. F., Mobashery S., Miller M. J., Eds.; Springer Nature: Cham, Switzerland, 2018; pp 165–188.
-
- Critically important antimicrobials for human medicine; World Health Organization, 2019. https://iris.who.int/bitstream/handle/10665/312266/9789241515528-eng.pdf... (accessed Oct 25, 2023).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

