Mapping the global interactome of the ARF family reveals spatial organization in cellular signaling pathways
- PMID: 38606629
- PMCID: PMC11166204
- DOI: 10.1242/jcs.262140
Mapping the global interactome of the ARF family reveals spatial organization in cellular signaling pathways
Abstract
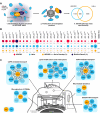
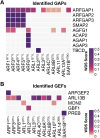
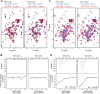
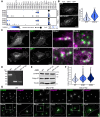
The ADP-ribosylation factors (ARFs) and ARF-like (ARL) GTPases serve as essential molecular switches governing a wide array of cellular processes. In this study, we used proximity-dependent biotin identification (BioID) to comprehensively map the interactome of 28 out of 29 ARF and ARL proteins in two cellular models. Through this approach, we identified ∼3000 high-confidence proximal interactors, enabling us to assign subcellular localizations to the family members. Notably, we uncovered previously undefined localizations for ARL4D and ARL10. Clustering analyses further exposed the distinctiveness of the interactors identified with these two GTPases. We also reveal that the expression of the understudied member ARL14 is confined to the stomach and intestines. We identified phospholipase D1 (PLD1) and the ESCPE-1 complex, more precisely, SNX1, as proximity interactors. Functional assays demonstrated that ARL14 can activate PLD1 in cellulo and is involved in cargo trafficking via the ESCPE-1 complex. Overall, the BioID data generated in this study provide a valuable resource for dissecting the complexities of ARF and ARL spatial organization and signaling.
Keywords: ARF GTPases; ARF-like GTPases, ARLs; BioID proteomics; ESCPE-1; Effector proteins; PLD1.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Update of
-
Mapping the global interactome of the ARF family reveals spatial organization in cellular signaling pathways.bioRxiv [Preprint]. 2024 Mar 25:2023.03.01.530598. doi: 10.1101/2023.03.01.530598. bioRxiv. 2024. Update in: J Cell Sci. 2024 May 1;137(9):jcs262140. doi: 10.1242/jcs.262140. PMID: 36909472 Free PMC article. Updated. Preprint.
References
-
- Abou Jamra, R., Philippe, O., Raas-Rothschild, A., Eck, S. H., Graf, E., Buchert, R., Borck, G., Ekici, A., Brockschmidt, F. F., Nothen, M. M.et al. (2011). Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature. Am. J. Hum. Genet. 88, 788-795. 10.1016/j.ajhg.2011.04.019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

