Treatment of post-treatment Lyme disease symptoms-a systematic review
- PMID: 38606630
- PMCID: PMC11235603
- DOI: 10.1111/ene.16293
Treatment of post-treatment Lyme disease symptoms-a systematic review
Abstract
Background and purpose: Residual symptoms after treatment of Lyme disease, sometimes called post-treatment Lyme disease symptoms (PTLDs), are a matter of ongoing controversy. To guide treatment recommendations, a systematic review was performed of the available literature on specific treatment for PTLDs.
Methods: A systematic literature search of MEDLINE and CENTRAL was performed. No restrictions on case definitions, study types or specific interventions were applied to enable a comprehensive overview of the available literature. Risk of bias was assessed using the Cochrane risk of bias tools for randomized controlled trials. Certainty of the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation approach. Outcomes of interest were quality of life, fatigue, depression and cognition as well as adverse events.
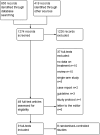
Results: After screening 1274 records, eight eligible randomized controlled trials were included. Heterogeneity was observed regarding inclusion criteria, intervention, length of treatment and outcome measures. For efficacy outcomes, results are presented narratively due to heterogeneity. Eligible studies show no statistically significant difference between antibiotics and placebo regarding quality of life, cognition and depression. Results for fatigue were inconsistent whilst studies with low risk of bias showed no statistically significant difference between antibiotics and placebo. Meta-analysis of safety outcomes showed statistically significantly more adverse events for antibiotics compared to placebo.
Conclusions: Available literature on treatment of PTLDs is heterogeneous, but overall shows evidence of no effect of antibiotics regarding quality of life, depression, cognition and fatigue whilst showing more adverse events. Patients with suspected PTLDs should not be treated with antibiotics.
Keywords: Lyme disease; Lyme neuroborreliosis; fatigue; post‐treatment Lyme disease syndrome; systematic review.
© 2024 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
R.D. reports lecture fees from Bayer, Alexion, Argenx, Merck, Novartis, Roche and Sanofi and consulting fees from Pfizer. G.T. reports no conflicts of interest. S.R. reports receiving consulting and lecture fees, and grant and research support from Baxter, Bayer Vital GmbH, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi‐Aventis and Teva. Furthermore, S.R. is a founding executive board member of Ravo Diagnostika GmbH, which sells in vitro diagnostic medical devices for the detection of infectious diseases (including Lyme neuroborreliosis) and paraneoplastic autoantibodies.
Figures
References
-
- Dersch R, Sommer H, Rauer S, Meerpohl JJ. Prevalence and spectrum of residual symptoms in Lyme neuroborreliosis after pharmacological treatment: a systematic review. J Neurol. 2016;263(1):17‐24. - PubMed
-
- Wormser GP, Weitzner E, McKenna D, et al. Long‐term assessment of health‐related quality of life in patients with culture‐confirmed early Lyme disease. Clin Infect Dis. 2015;61(2):244‐247. - PubMed
-
- Asch ES, Bujak DI, Weiss M, Peterson MG, Weinstein A. Lyme disease: an infectious and postinfectious syndrome. J Rheumatol. 1994;21(3):454‐461. - PubMed
-
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089‐1134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical