Current treatment strategies for ovarian cancer in the East Asian Gynecologic Oncology Trial Group (EAGOT)
- PMID: 38606827
- PMCID: PMC11107282
- DOI: 10.3802/jgo.2024.35.e87
Current treatment strategies for ovarian cancer in the East Asian Gynecologic Oncology Trial Group (EAGOT)
Abstract
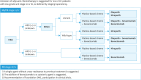
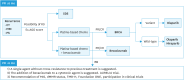
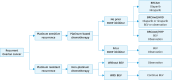
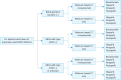
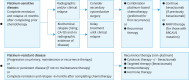
Ovarian cancer, notable for its severe prognosis among gynecologic cancers, has seen substantial progress in treatment approaches recently. Enhanced protocols in chemotherapy and the introduction of poly (ADP-ribose) polymerase (PARP) inhibitors for maintenance therapy have markedly improved outcomes for patients with specific genetic profiles, such as those positive for BRCA mutations or exhibiting homologous recombination deficiency (HRD). Additionally, the method of intraperitoneal chemotherapy administration has emerged as a valuable alternative to traditional transvenous routes, showing promise for wider clinical adoption. The field of surgery has also evolved, with increasing exploration into the benefits and feasibility of laparoscopic methods over more invasive traditional surgeries, aiming for complete tumor removal but with reduced patient impact. The hereditary nature of ovarian cancer underscores the importance of genetic testing, which has become integral in tailoring treatment strategies, particularly in determining suitability for PARP inhibitors. The formation of the East Asian Gynecologic Oncology Trial Group (EAGOT) aims to optimize treatment across Japan, Korea, China, and Taiwan. The ovarian cancer committee of EAGOT shared the current policies, focusing on 5 topics: 1) strategies for maintenance therapy after initial surgery and chemotherapy, 2) drug regimens for platinum-sensitive and platinum-resistant recurrence, 3) intraperitoneal chemotherapy, 4) laparoscopic surgery as an alternative to laparotomy, and 5) current status of genetic testing (BRCA, HRD, and panel tests) for ovarian cancer and its prospects. EAGOT's multi-national trials aim to harmonize these evolving treatment strategies, ensuring that the latest and most effective protocols are accessible across the region, thereby significantly impacting patient outcomes in East Asia.
Keywords: East Asian Gynecologic Oncology Trial Group; Genetic Testing; Intraperitoneal Chemotherapy; Laparoscopic Surgery; Ovarian Cancer; PARP Inhibitors.
© 2024. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



References
-
- Trimbos JB, Parmar M, Vergote I, Guthrie D, Bolis G, Colombo N, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst. 2003;95:105–112. - PubMed
-
- Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C, et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl Cancer Inst. 2003;95:113–125. - PubMed
-
- Tew WP, Lacchetti C, Kohn EC PARP Inhibitors in the Management of Ovarian Cancer Guideline Expert Panel. Poly(ADP-ribose) polymerase inhibitors in the management of ovarian cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2022;40:3878–3881. - PubMed

