Respiratory Syncytial Virus Prefusion F Vaccination: Antibody Persistence and Revaccination
- PMID: 38606958
- PMCID: PMC11481295
- DOI: 10.1093/infdis/jiae185
Respiratory Syncytial Virus Prefusion F Vaccination: Antibody Persistence and Revaccination
Abstract
Background: Respiratory syncytial virus (RSV) causes substantial respiratory disease. Bivalent RSV prefusion F (RSVpreF) vaccine is licensed in ≥60-year-olds. RSVpreF was well tolerated and immunogenic in a phase 1/2 study. We evaluated antibody persistence after initial vaccination and safety and immunogenicity after revaccination from this study.
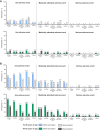
Methods: Healthy adults were randomized to receive initial vaccination and revaccination 12 months later with either placebo or RSVpreF (240 µg with or without aluminum hydroxide). RSV-A and RSV-B geometric mean neutralizing titers (GMTs) were measured through 12 months after both vaccinations. Tolerability and safety were assessed.
Results: There were 263 participants revaccinated (18-49 years old, n = 134; 65-85 years old, n = 129). Among 18- to 49-year-olds and 65- to 85-year-olds, geometric mean fold rises (GMFRs) for both RSV subgroups (RSV-A, RSV-B) 1 month after initial RSVpreF vaccination were 13.3 to 20.4 and 8.9 to 15.5, respectively, as compared with levels before initial vaccination; corresponding GMFRs 12 months after initial vaccination were 4.1 to 5.0 and 2.6 to 4.1. GMFRs 1 month after revaccination vs levels before revaccination were 1.4 to 2.3 and 1.4 to 2.2 for 18- to 49-year-olds and 65- to 85-year-olds. Peak GMTs after revaccination were lower than those after initial vaccination. GMTs 12 months after initial vaccination and revaccination were similar, with GMFRs ranging from 0.7 to 1.6. No safety signals occurred.
Conclusions: RSVpreF revaccination was immunogenic and well tolerated among adults. Clinical Trials Registration. NCT03529773 (ClinicalTrials.gov).
Keywords: RSVpreF; immunogenicity; neutralizing responses; respiratory syncytial virus; safety; vaccine.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. E. E. W. reports receiving grants from Pfizer, Merck Sharp and Dohme, and Janssen and serving in unpaid consultancy roles for Janssen, Merck, Moderna, and Pfizer. A. R. F. reports receiving grant funding from Pfizer Inc, Merck, Janssen, BioFire Diagnostics, Vax Co, and CyanVac and consulting fees from Gilead, GlaxoSmithKline, Icosavax, and Novavax. A. M. Z., Q. J., A. G., D. R., E. G., D. C., K. U. J., W. C. G., K. A. S., and B. S.-T. are current or former employees of Pfizer Inc and may hold stock or stock options. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures





References
-
- Li Y, Johnson EK, Shi T, et al. . National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med 2021; 9:175–85. - PubMed
-
- Branche AR, Saiman L, Walsh EE, et al. . Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis 2022; 74:1004–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

