Nasal septum-derived chondroprogenitor cells control mandibular condylar resorption consequent to orthognathic surgery: a clinical trial
- PMID: 38606986
- PMCID: PMC11227969
- DOI: 10.1093/stcltm/szae026
Nasal septum-derived chondroprogenitor cells control mandibular condylar resorption consequent to orthognathic surgery: a clinical trial
Abstract
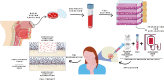
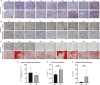
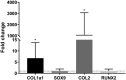
Condylar resorption is an aggressive and disability form of temporomandibular joint (TMJ) degenerative disease, usually non-respondent to conservative or minimally invasive therapies and often leading to surgical intervention and prostheses implantation. This condition is also one of the most dreaded postoperative complications of orthognathic surgery, with severe cartilage erosion and loss of subchondral bone volume and mineral density, associated with a painful or not inflammatory processes. Because regenerative medicine has emerged as an alternative for orthopedic cases with advanced degenerative joint disease, we conducted a phase I/IIa clinical trial (U1111-1194-6997) to evaluate the safety and efficacy of autologous nasal septal chondroprogenitor cells. Ten participants underwent biopsy of the nasal septum cartilage during their orthognathic surgery. The harvested cells were cultured in vitro and analyzed for viability, presence of phenotype markers for mesenchymal stem and/or chondroprogenitor cells, and the potential to differentiate into chondrocytes, adipocytes, and osteoblasts. After the intra-articular injection of the cell therapy, clinical follow-up was performed using the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) and computed tomography (CT) images. No serious adverse events related to the cell therapy injection were observed during the 12-month follow-up period. It was found that autologous chondroprogenitors reduced arthralgia, promoted stabilization of mandibular function and condylar volume, and regeneration of condylar tissues. This study demonstrates that chondroprogenitor cells from the nasal septum may be a promise strategy for the treatment of temporomandibular degenerative joint disease that do not respond to other conservative therapies.
Keywords: cellular therapy; clinical translational; progenitor cells; skeleton.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures

Similar articles
-
Temporomandibular joint regeneration: proposal of a novel treatment for condylar resorption after orthognathic surgery using transplantation of autologous nasal septum chondrocytes, and the first human case report.Stem Cell Res Ther. 2018 Apr 7;9(1):94. doi: 10.1186/s13287-018-0806-4. Stem Cell Res Ther. 2018. PMID: 29625584 Free PMC article.
-
Differential effects of high-physiological oestrogen on the degeneration of mandibular condylar cartilage and subchondral bone.Bone. 2018 Jun;111:9-22. doi: 10.1016/j.bone.2018.03.008. Epub 2018 Mar 10. Bone. 2018. PMID: 29530720
-
Norepinephrine Regulates Condylar Bone Loss via Comorbid Factors.J Dent Res. 2015 Jun;94(6):813-20. doi: 10.1177/0022034515577677. Epub 2015 Mar 12. J Dent Res. 2015. PMID: 25818584
-
Condylar resorptions and orthodontic-surgical treatment: State of the art.Int Orthod. 2016 Dec;14(4):503-527. doi: 10.1016/j.ortho.2016.10.011. Epub 2016 Nov 18. Int Orthod. 2016. PMID: 27867065 Review.
-
Adolescent internal condylar resorption (AICR) of the temporomandibular joint, part 1: A review for diagnosis and treatment considerations.Cranio. 2019 Jan;37(1):35-44. doi: 10.1080/08869634.2017.1386752. Epub 2017 Nov 10. Cranio. 2019. PMID: 29125402 Review.
Cited by
-
Differentiation of stem cells into chondrocytes and their potential clinical application in cartilage regeneration.Histochem Cell Biol. 2025 Jan 25;163(1):27. doi: 10.1007/s00418-025-02356-7. Histochem Cell Biol. 2025. PMID: 39863760 Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

