Ubiquitinome Analysis Uncovers Alterations in Synaptic Proteins and Glucose Metabolism Enzymes in the Hippocampi of Adolescent Mice Following Cold Exposure
- PMID: 38607009
- PMCID: PMC11011669
- DOI: 10.3390/cells13070570
Ubiquitinome Analysis Uncovers Alterations in Synaptic Proteins and Glucose Metabolism Enzymes in the Hippocampi of Adolescent Mice Following Cold Exposure
Abstract
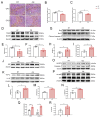
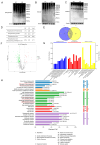
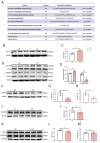
Cold exposure exerts negative effects on hippocampal nerve development in adolescent mice, but the underlying mechanisms are not fully understood. Given that ubiquitination is essential for neurodevelopmental processes, we attempted to investigate the effects of cold exposure on the hippocampus from the perspective of ubiquitination. By conducting a ubiquitinome analysis, we found that cold exposure caused changes in the ubiquitination levels of a variety of synaptic-associated proteins. We validated changes in postsynaptic density-95 (PSD-95) ubiquitination levels by immunoprecipitation, revealing reductions in both the K48 and K63 polyubiquitination levels of PSD-95. Golgi staining further demonstrated that cold exposure decreased the dendritic-spine density in the CA1 and CA3 regions of the hippocampus. Additionally, bioinformatics analysis revealed that differentially ubiquitinated proteins were enriched in the glycolytic, hypoxia-inducible factor-1 (HIF-1), and 5'-monophosphate (AMP)-activated protein kinase (AMPK) pathways. Protein expression analysis confirmed that cold exposure activated the mammalian target of rapamycin (mTOR)/HIF-1α pathway. We also observed suppression of pyruvate kinase M2 (PKM2) protein levels and the pyruvate kinase (PK) activity induced by cold exposure. Regarding oxidative phosphorylation, a dramatic decrease in mitochondrial respiratory-complex I activity was observed, along with reduced gene expression of the key subunits NADH: ubiquinone oxidoreductase core subunit V1 (Ndufv1) and Ndufv2. In summary, cold exposure negatively affects hippocampal neurodevelopment and causes abnormalities in energy homeostasis within the hippocampus.
Keywords: PSD-95; cold exposure; glucose metabolism; hippocampus; ubiquitinome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Proteasome-independent polyubiquitin linkage regulates synapse scaffolding, efficacy, and plasticity.Proc Natl Acad Sci U S A. 2017 Oct 10;114(41):E8760-E8769. doi: 10.1073/pnas.1620153114. Epub 2017 Sep 25. Proc Natl Acad Sci U S A. 2017. PMID: 28973854 Free PMC article.
-
Prenatal stress increased Snk Polo-like kinase 2, SCF β-TrCP ubiquitin ligase and ubiquitination of SPAR in the hippocampus of the offspring at adulthood.Int J Dev Neurosci. 2013 Nov;31(7):560-7. doi: 10.1016/j.ijdevneu.2013.06.011. Epub 2013 Jul 10. Int J Dev Neurosci. 2013. PMID: 23850969
-
Pyruvate kinase M knockdown-induced signaling via AMP-activated protein kinase promotes mitochondrial biogenesis, autophagy, and cancer cell survival.J Biol Chem. 2017 Sep 15;292(37):15561-15576. doi: 10.1074/jbc.M117.791343. Epub 2017 Aug 4. J Biol Chem. 2017. PMID: 28778925 Free PMC article.
-
The mTOR Pathway Regulates PKM2 to Affect Glycolysis in Esophageal Squamous Cell Carcinoma.Technol Cancer Res Treat. 2018 Jan 1;17:1533033818780063. doi: 10.1177/1533033818780063. Technol Cancer Res Treat. 2018. PMID: 29916308 Free PMC article.
-
Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells.Int J Biochem Cell Biol. 2011 Jul;43(7):969-80. doi: 10.1016/j.biocel.2010.02.005. Epub 2010 Feb 13. Int J Biochem Cell Biol. 2011. PMID: 20156581 Review.
Cited by
-
Cypin regulates K63-linked polyubiquitination to shape synaptic content.Sci Adv. 2025 Jul 11;11(28):eads5467. doi: 10.1126/sciadv.ads5467. Epub 2025 Jul 11. Sci Adv. 2025. PMID: 40644549 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

