NF-κB and JAK/STAT Signaling Pathways as Crucial Regulators of Neuroinflammation and Astrocyte Modulation in Spinal Cord Injury
- PMID: 38607020
- PMCID: PMC11011519
- DOI: 10.3390/cells13070581
NF-κB and JAK/STAT Signaling Pathways as Crucial Regulators of Neuroinflammation and Astrocyte Modulation in Spinal Cord Injury
Abstract
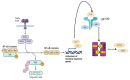
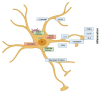
Spinal cord injury (SCI) leads to significant functional impairments below the level of the injury, and astrocytes play a crucial role in the pathophysiology of SCI. Astrocytes undergo changes and form a glial scar after SCI, which has traditionally been viewed as a barrier to axonal regeneration and functional recovery. Astrocytes activate intracellular signaling pathways, including nuclear factor κB (NF-κB) and Janus kinase-signal transducers and activators of transcription (JAK/STAT), in response to external stimuli. NF-κB and STAT3 are transcription factors that play a pivotal role in initiating gene expression related to astrogliosis. The JAK/STAT signaling pathway is essential for managing secondary damage and facilitating recovery processes post-SCI: inflammation, glial scar formation, and astrocyte survival. NF-κB activation in astrocytes leads to the production of pro-inflammatory factors by astrocytes. NF-κB and STAT3 signaling pathways are interconnected: NF-κB activation in astrocytes leads to the release of interleukin-6 (IL-6), which interacts with the IL-6 receptor and initiates STAT3 activation. By modulating astrocyte responses, these pathways offer promising avenues for enhancing recovery outcomes, illustrating the crucial need for further investigation into their mechanisms and therapeutic applications in SCI treatment.
Keywords: JAK/STAT; NF-κB; astrocyte; glial scar; spinal cord injury.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

