Oral Antiviral Defense: Saliva- and Beverage-like Hypotonicity Dynamically Regulate Formation of Membraneless Biomolecular Condensates of Antiviral Human MxA in Oral Epithelial Cells
- PMID: 38607029
- PMCID: PMC11011872
- DOI: 10.3390/cells13070590
Oral Antiviral Defense: Saliva- and Beverage-like Hypotonicity Dynamically Regulate Formation of Membraneless Biomolecular Condensates of Antiviral Human MxA in Oral Epithelial Cells
Abstract
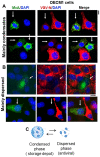
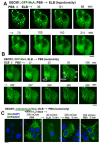
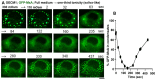
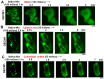
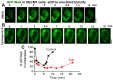
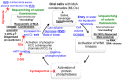
The oral mucosa represents a defensive barrier between the external environment and the rest of the body. Oral mucosal cells are constantly bathed in hypotonic saliva (normally one-third tonicity compared to plasma) and are repeatedly exposed to environmental stresses of tonicity, temperature, and pH by the drinks we imbibe (e.g., hypotonic: water, tea, and coffee; hypertonic: assorted fruit juices, and red wines). In the mouth, the broad-spectrum antiviral mediator MxA (a dynamin-family large GTPase) is constitutively expressed in healthy periodontal tissues and induced by Type III interferons (e.g., IFN-λ1/IL-29). Endogenously induced human MxA and exogenously expressed human GFP-MxA formed membraneless biomolecular condensates in the cytoplasm of oral carcinoma cells (OECM1 cell line). These condensates likely represent storage granules in equilibrium with antivirally active dispersed MxA. Remarkably, cytoplasmic MxA condensates were exquisitely sensitive sensors of hypotonicity-the condensates in oral epithelium disassembled within 1-2 min of exposure of cells to saliva-like one-third hypotonicity, and spontaneously reassembled in the next 4-7 min. Water, tea, and coffee enhanced this disassembly. Fluorescence changes in OECM1 cells preloaded with calcein-AM (a reporter of cytosolic "macromolecular crowding") confirmed that this process involved macromolecular uncrowding and subsequent recrowding secondary to changes in cell volume. However, hypertonicity had little effect on MxA condensates. The spontaneous reassembly of GFP-MxA condensates in oral epithelial cells, even under continuous saliva-like hypotonicity, was slowed by the protein-phosphatase-inhibitor cyclosporin A (CsA) and by the K-channel-blocker tetraethylammonium chloride (TEA); this is suggestive of the involvement of the volume-sensitive WNK kinase-protein phosphatase (PTP)-K-Cl cotransporter (KCC) pathway in the regulated volume decrease (RVD) during condensate reassembly in oral cells. The present study identifies a novel subcellular consequence of hypotonic stress in oral epithelial cells, in terms of the rapid and dynamic changes in the structure of one class of phase-separated biomolecular condensates in the cytoplasm-the antiviral MxA condensates. More generally, the data raise the possibility that hypotonicity-driven stresses likely affect other intracellular functions involving liquid-liquid phase separation (LLPS) in cells of the oral mucosa.
Keywords: barrier immunity; biomolecular condensates; environmental hypotonic stress; human myxovirus resistance protein (MxA); interferon-λ/IL-29; macromolecular crowding; membraneless organelles (MLOs); oral/gingival epithelium; osmoregulation; regulated volume decrease (RVD).
Conflict of interest statement
The authors declare no conflicts of interest. New York Medical College had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Temperature and WNK-SPAK/OSR1 Kinases Dynamically Regulate Antiviral Human GFP-MxA Biomolecular Condensates in Oral Cancer Cells.Cells. 2025 Jun 20;14(13):947. doi: 10.3390/cells14130947. Cells. 2025. PMID: 40643468 Free PMC article.
-
Rapid Reversible Osmoregulation of Cytoplasmic Biomolecular Condensates of Human Interferon-α-Induced Antiviral MxA GTPase.Int J Mol Sci. 2022 Oct 22;23(21):12739. doi: 10.3390/ijms232112739. Int J Mol Sci. 2022. PMID: 36361529 Free PMC article.
-
Human Antiviral Protein MxA Forms Novel Metastable Membraneless Cytoplasmic Condensates Exhibiting Rapid Reversible Tonicity-Driven Phase Transitions.J Virol. 2019 Oct 29;93(22):e01014-19. doi: 10.1128/JVI.01014-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31484749 Free PMC article.
-
Metastable biomolecular condensates of interferon-inducible antiviral Mx-family GTPases: A paradigm shift in the last three years.J Biosci. 2021;46(3):72. doi: 10.1007/s12038-021-00187-x. J Biosci. 2021. PMID: 34323222 Free PMC article. Review.
-
Biomolecular condensates in cell biology and virology: Phase-separated membraneless organelles (MLOs).Anal Biochem. 2020 May 15;597:113691. doi: 10.1016/j.ab.2020.113691. Epub 2020 Mar 16. Anal Biochem. 2020. PMID: 32194074 Review.
Cited by
-
Temperature and WNK-SPAK/OSR1 Kinases Dynamically Regulate Antiviral Human GFP-MxA Biomolecular Condensates in Oral Cancer Cells.Cells. 2025 Jun 20;14(13):947. doi: 10.3390/cells14130947. Cells. 2025. PMID: 40643468 Free PMC article.
References
-
- Rodriguez-Hernandez C.J., Sokoloski K.J., Stocke K.S., Dukka H., Jin S., Metzler M.A., Zaitsev K., Shpak B., Shen D., Miller D.P., et al. Microbiome-mediated incapacitation of interferon lambda production in the oral mucosa. Proc. Natl. Acad. Sci. USA. 2021;118:e2105170118. doi: 10.1073/pnas.2105170118. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

