Sustained Retinal Defocus Increases the Effect of Induced Myopia on the Retinal Astrocyte Template
- PMID: 38607034
- PMCID: PMC11011523
- DOI: 10.3390/cells13070595
Sustained Retinal Defocus Increases the Effect of Induced Myopia on the Retinal Astrocyte Template
Abstract
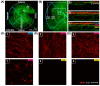
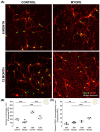
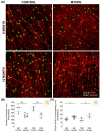
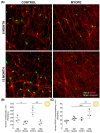
The aim of this article is to describe sustained myopic eye growth's effect on astrocyte cellular distribution and its association with inner retinal layer thicknesses. Astrocyte density and distribution, retinal nerve fiber layer (RNFL), ganglion cell layer, and inner plexiform layer (IPL) thicknesses were assessed using immunochemistry and spectral-domain optical coherence tomography on seventeen common marmoset retinas (Callithrix jacchus): six induced with myopia from 2 to 6 months of age (6-month-old myopes), three induced with myopia from 2 to 12 months of age (12-month-old myopes), five age-matched 6-month-old controls, and three age-matched 12-month-old controls. Untreated marmoset eyes grew normally, and both RNFL and IPL thicknesses did not change with age, with astrocyte numbers correlating to RNFL and IPL thicknesses in both control age groups. Myopic marmosets did not follow this trend and, instead, exhibited decreased astrocyte density, increased GFAP+ spatial coverage, and thinner RNFL and IPL, all of which worsened over time. Myopic changes in astrocyte density, GFAP+ spatial coverage and inner retinal layer thicknesses suggest astrocyte template reorganization during myopia development and progression which increased over time. Whether or not these changes are constructive or destructive to the retina still remains to be assessed.
Keywords: aging; astrocytes; marmoset; myopia; neurovascular unit.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Experimental Myopia Results in Peripapillary Ganglion Cell and Astrocyte Reorganization with No Functional Implications During Early Development.Int J Mol Sci. 2024 Dec 16;25(24):13484. doi: 10.3390/ijms252413484. Int J Mol Sci. 2024. PMID: 39769248 Free PMC article.
-
Sustained Experimental Myopia Exacerbates the Effect of Eye Growth on Retinal Ganglion Cell Density and Function.Int J Mol Sci. 2025 Mar 20;26(6):2824. doi: 10.3390/ijms26062824. Int J Mol Sci. 2025. PMID: 40141465 Free PMC article.
-
Myopia Alters the Structural Organization of the Retinal Vasculature, GFAP-Positive Glia, and Ganglion Cell Layer Thickness.Int J Mol Sci. 2022 May 31;23(11):6202. doi: 10.3390/ijms23116202. Int J Mol Sci. 2022. PMID: 35682880 Free PMC article.
-
Evaluation of the Ganglion Cell Complex and Retinal Nerve Fiber Layer in Low, Moderate, and High Myopia: A Study by RTVue Spectral Domain Optical Coherence Tomography.Semin Ophthalmol. 2017;32(6):682-688. doi: 10.3109/08820538.2016.1170157. Epub 2016 Jul 12. Semin Ophthalmol. 2017. PMID: 27404600
-
Ganglion cell-inner plexiform layer and retinal nerve fiber layer thickness according to myopia and optic disc area: a quantitative and three-dimensional analysis.BMC Ophthalmol. 2017 Mar 11;17(1):22. doi: 10.1186/s12886-017-0419-1. BMC Ophthalmol. 2017. PMID: 28283025 Free PMC article.
Cited by
-
Retinal glia in myopia: current understanding and future directions.Front Cell Dev Biol. 2024 Dec 20;12:1512988. doi: 10.3389/fcell.2024.1512988. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39759766 Free PMC article. Review.
-
Experimental Myopia Results in Peripapillary Ganglion Cell and Astrocyte Reorganization with No Functional Implications During Early Development.Int J Mol Sci. 2024 Dec 16;25(24):13484. doi: 10.3390/ijms252413484. Int J Mol Sci. 2024. PMID: 39769248 Free PMC article.
-
Sustained Experimental Myopia Exacerbates the Effect of Eye Growth on Retinal Ganglion Cell Density and Function.Int J Mol Sci. 2025 Mar 20;26(6):2824. doi: 10.3390/ijms26062824. Int J Mol Sci. 2025. PMID: 40141465 Free PMC article.
References
-
- Curtin B.J. The Myopias: Basic Science and Clinical Management. Harper & Row; Philadelphia, PA, USA: 1985.
-
- Flitcroft D.I., He M., Jonas J.B., Jong M., Naidoo K., Ohno-Matsui K., Rahi J., Resnikoff S., Vitale S., Yannuzzi L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019;60:M20–M30. doi: 10.1167/iovs.18-25957. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

