Genetic Signature of Human Pancreatic Cancer and Personalized Targeting
- PMID: 38607041
- PMCID: PMC11011857
- DOI: 10.3390/cells13070602
Genetic Signature of Human Pancreatic Cancer and Personalized Targeting
Abstract
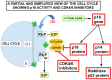
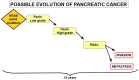
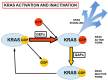
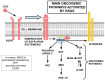
Pancreatic cancer is a highly lethal disease with a 5-year survival rate of around 11-12%. Surgery, being the treatment of choice, is only possible in 20% of symptomatic patients. The main reason is that when it becomes symptomatic, IT IS the tumor is usually locally advanced and/or has metastasized to distant organs; thus, early diagnosis is infrequent. The lack of specific early symptoms is an important cause of late diagnosis. Unfortunately, diagnostic tumor markers become positive at a late stage, and there is a lack of early-stage markers. Surgical and non-surgical cases are treated with neoadjuvant and/or adjuvant chemotherapy, and the results are usually poor. However, personalized targeted therapy directed against tumor drivers may improve this situation. Until recently, many pancreatic tumor driver genes/proteins were considered untargetable. Chemical and physical characteristics of mutated KRAS are a formidable challenge to overcome. This situation is slowly changing. For the first time, there are candidate drugs that can target the main driver gene of pancreatic cancer: KRAS. Indeed, KRAS inhibition has been clinically achieved in lung cancer and, at the pre-clinical level, in pancreatic cancer as well. This will probably change the very poor outlook for this disease. This paper reviews the genetic characteristics of sporadic and hereditary predisposition to pancreatic cancer and the possibilities of a personalized treatment according to the genetic signature.
Keywords: KRAS; PDAC (pancreatic ductal adenocarcinoma); driver mutations; personalized treatment.
Conflict of interest statement
The authors declare no conflicts of interest nor any potential commercial interests.
Figures
Similar articles
-
Mutations in key driver genes of pancreatic cancer: molecularly targeted therapies and other clinical implications.Acta Pharmacol Sin. 2021 Nov;42(11):1725-1741. doi: 10.1038/s41401-020-00584-2. Epub 2021 Feb 11. Acta Pharmacol Sin. 2021. PMID: 33574569 Free PMC article. Review.
-
Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: Hopes and realities.Eur J Cancer. 2016 Feb;54:75-83. doi: 10.1016/j.ejca.2015.11.012. Epub 2015 Dec 28. Eur J Cancer. 2016. PMID: 26735353 Review.
-
Mutant KRAS Circulating Tumor DNA Is an Accurate Tool for Pancreatic Cancer Monitoring.Oncologist. 2018 May;23(5):566-572. doi: 10.1634/theoncologist.2017-0467. Epub 2018 Jan 25. Oncologist. 2018. PMID: 29371474 Free PMC article.
-
Targeting KRAS in pancreatic cancer.Oncol Res. 2024 Apr 23;32(5):799-805. doi: 10.32604/or.2024.045356. eCollection 2024. Oncol Res. 2024. PMID: 38686056 Free PMC article. Review.
-
The type of KRAS mutation drives PI3Kα/γ signalling dependency: Implication for the choice of targeted therapy in pancreatic adenocarcinoma patients.Clin Res Hepatol Gastroenterol. 2021 Jan;45(1):101473. doi: 10.1016/j.clinre.2020.05.021. Epub 2020 Jun 24. Clin Res Hepatol Gastroenterol. 2021. PMID: 32593694
Cited by
-
Molecular Targets for the Diagnosis and Treatment of Pancreatic Cancer.Int J Mol Sci. 2024 Oct 9;25(19):10843. doi: 10.3390/ijms251910843. Int J Mol Sci. 2024. PMID: 39409171 Free PMC article. Review.
-
Precision Targeting Strategies in Pancreatic Cancer: The Role of Tumor Microenvironment.Cancers (Basel). 2024 Aug 19;16(16):2876. doi: 10.3390/cancers16162876. Cancers (Basel). 2024. PMID: 39199647 Free PMC article. Review.
-
Digestive cancers: mechanisms, therapeutics and management.Signal Transduct Target Ther. 2025 Jan 15;10(1):24. doi: 10.1038/s41392-024-02097-4. Signal Transduct Target Ther. 2025. PMID: 39809756 Free PMC article. Review.
-
Integrative single-cell RNA sequencing and bulk RNA sequencing reveals the characteristics of glutathione metabolism and protective role of GSTA4 gene in pancreatic cancer.Front Immunol. 2025 May 1;16:1571431. doi: 10.3389/fimmu.2025.1571431. eCollection 2025. Front Immunol. 2025. PMID: 40375987 Free PMC article.
-
Consensus, debate, and prospective on pancreatic cancer treatments.J Hematol Oncol. 2024 Oct 10;17(1):92. doi: 10.1186/s13045-024-01613-x. J Hematol Oncol. 2024. PMID: 39390609 Free PMC article. Review.
References
-
- Subbiah V., Puzanov I., Blay J.-Y., Chau I., Lockhart A.C., Raje N.S., Wolf J., Baselga J., Meric-Bernstam F., Roszik J., et al. Pan-Cancer Efficacy of Vemurafenib in BRAFV600-Mutant Non-Melanoma Cancers. Cancer Discov. 2020;10:657–663. doi: 10.1158/2159-8290.CD-19-1265. - DOI - PMC - PubMed
-
- Kong B., Thoma E., Michalski C.W. From Tissue Turnover to the Cell of Origin of Pancreatic Cancer. In: Beger H.G., Büchler M.W., Hruban R.H., Mayerle J., Neoptolemos J.P., Shimosegawa T., Warshaw A.L., Whitcomb D.C., Zhao Y., Groß C., editors. The Pancreas. Publisher Wiley; Hoboken, NJ, USA: 2023. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

