The Olfactory Trail of Neurodegenerative Diseases
- PMID: 38607054
- PMCID: PMC11012126
- DOI: 10.3390/cells13070615
The Olfactory Trail of Neurodegenerative Diseases
Abstract
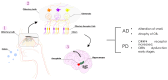
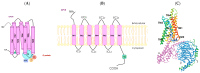
Alterations in olfactory functions are proposed as possible early biomarkers of neurodegenerative diseases. Parkinson's and Alzheimer's diseases manifest olfactory dysfunction as a symptom, which is worth mentioning. The alterations do not occur in all patients, but they can serve to rule out neurodegenerative pathologies that are not associated with small deficits. Several prevalent neurodegenerative conditions, including impaired smell, arise in the early stages of Parkinson's and Alzheimer's diseases, presenting an attractive prospect as a snitch for early diagnosis. This review covers the current knowledge on the link between olfactory deficits and Parkinson's and Alzheimer's diseases. The review also covers the emergence of olfactory receptors as actors in the pathophysiology of these diseases. Olfactory receptors are not exclusively expressed in olfactory sensory neurons. Olfactory receptors are widespread in the human body; they are expressed, among others, in the testicles, lungs, intestines, kidneys, skin, heart, and blood cells. Although information on these ectopically expressed olfactory receptors is limited, they appear to be involved in cell recognition, migration, proliferation, wound healing, apoptosis, and exocytosis. Regarding expression in non-chemosensory regions of the central nervous system (CNS), future research should address the role, in both the glia and neurons, of olfactory receptors. Here, we review the limited but relevant information on the altered expression of olfactory receptor genes in Parkinson's and Alzheimer's diseases. By unraveling how olfactory receptor activation is involved in neurodegeneration and identifying links between olfactory structures and neuronal death, valuable information could be gained for early diagnosis and intervention strategies in neurodegenerative diseases.
Keywords: Alzheimer’s disease; Parkinson’s disease; ectopic expression; microglia; neurons; olfactory receptors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Olfactory Dysfunction in Neurodegenerative Diseases.Curr Allergy Asthma Rep. 2018 Jun 15;18(8):42. doi: 10.1007/s11882-018-0796-4. Curr Allergy Asthma Rep. 2018. PMID: 29904888 Review.
-
The human olfactory system in two proteinopathies: Alzheimer's and Parkinson's diseases.Transl Neurodegener. 2020 Jun 3;9(1):22. doi: 10.1186/s40035-020-00200-7. Transl Neurodegener. 2020. PMID: 32493457 Free PMC article. Review.
-
Neurons, Nose, and Neurodegenerative Diseases: Olfactory Function and Cognitive Impairment.Int J Mol Sci. 2023 Jan 20;24(3):2117. doi: 10.3390/ijms24032117. Int J Mol Sci. 2023. PMID: 36768440 Free PMC article. Review.
-
Animal models of olfactory dysfunction in neurodegenerative diseases.Handb Clin Neurol. 2019;164:431-452. doi: 10.1016/B978-0-444-63855-7.00024-1. Handb Clin Neurol. 2019. PMID: 31604561 Review.
-
[Changes in olfaction during ageing and in certain neurodegenerative diseases: up-to-date].Rev Med Interne. 2015 Jan;36(1):31-7. doi: 10.1016/j.revmed.2014.09.008. Epub 2014 Oct 7. Rev Med Interne. 2015. PMID: 25304170 Review. French.
Cited by
-
Diagnostic value of olfactory function testing for Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis.Front Aging Neurosci. 2025 May 15;17:1551939. doi: 10.3389/fnagi.2025.1551939. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40443794 Free PMC article.
-
Preliminary Findings on the Morphometric Characteristics of the Olfactory Bulb in the Cat.Animals (Basel). 2024 Dec 12;14(24):3590. doi: 10.3390/ani14243590. Animals (Basel). 2024. PMID: 39765495 Free PMC article.
-
Nasal microbiome in relation to olfactory dysfunction and cognitive decline in older adults.Transl Psychiatry. 2025 Apr 4;15(1):122. doi: 10.1038/s41398-025-03346-y. Transl Psychiatry. 2025. PMID: 40185726 Free PMC article.
-
Firing Patterns of Mitral Cells and Their Transformation in the Main Olfactory Bulb.Brain Sci. 2024 Jul 3;14(7):678. doi: 10.3390/brainsci14070678. Brain Sci. 2024. PMID: 39061419 Free PMC article.
-
Olfactory receptors and human diseases.Cell Tissue Res. 2025 Jul;401(1):1-14. doi: 10.1007/s00441-025-03971-5. Epub 2025 Apr 25. Cell Tissue Res. 2025. PMID: 40278904 Review.
References
-
- Cartas-Cejudo P., Lachén-Montes M., Ferrer I., Fernández-Irigoyen J., Santamaría E. Sex-divergent effects on the NAD+-dependent deacetylase sirtuin signaling across the olfactory–entorhinal–amygdaloid axis in Alzheimer’s and Parkinson’s diseases. Biol. Sex Differ. 2023;14:5. doi: 10.1186/s13293-023-00487-x. - DOI - PMC - PubMed
-
- Loftus B.D., Athni S.S., Cherches I.M. Neurology Secrets. Elsevier/Mosby; Amsterdam, The Netherlands: 2010. Clinical Neuroanatomy; pp. 18–54. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

