The Natural Product Secoemestrin C Inhibits Colorectal Cancer Stem Cells via p38-S100A8 Feed-Forward Regulatory Loop
- PMID: 38607060
- PMCID: PMC11011747
- DOI: 10.3390/cells13070620
The Natural Product Secoemestrin C Inhibits Colorectal Cancer Stem Cells via p38-S100A8 Feed-Forward Regulatory Loop
Abstract
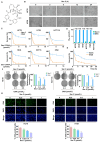
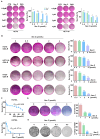
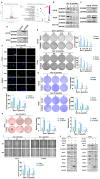
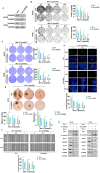
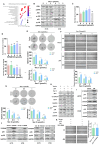
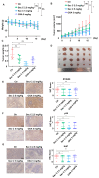
Cancer stem cells (CSCs) are closely associated with tumor initiation, metastasis, chemoresistance, and recurrence, which represent some of the primary obstacles to cancer treatment. Targeting CSCs has become an important therapeutic approach to cancer care. Secoemestrin C (Sec C) is a natural compound with strong anti-tumor activity and low toxicity. Here, we report that Sec C effectively inhibited colorectal CSCs and non-CSCs concurrently, mainly by inhibiting proliferation, self-renewal, metastasis, and drug resistance. Mechanistically, RNA-seq analysis showed that the pro-inflammation pathway of the IL17 axis was enriched, and its effector S100A8 was dramatically decreased in Sec C-treated cells, whose roles in the stemness of CSCs have not been fully clarified. We found that the overexpression of S100A8 hindered the anti-CSCs effect of Sec C, and S100A8 deficiency attenuated the stemness traits of CSCs to enhance the Sec C killing activity on them. Meanwhile, the p38 signal pathway, belonging to the IL17 downstream axis, can also mediate CSCs and counter with Sec C. Notably, we found that S100A8 upregulation increased the p38 protein level, and p38, in turn, promoted S100A8 expression. This indicated that p38 may have a mutual feedback loop with S100A8. Our study discovered that Sec C was a powerful anti-colorectal CSC agent, and that the positive feedback loop of p38-S100A8 mediated Sec C activity. This showed that Sec C could act as a promising clinical candidate in colorectal cancer treatment, and S100A8 could be a prospective drug target.
Keywords: CSCs; S100A8; colorectal cancer; p38; secoemestrin C; stemness.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Yang I.P., Tsai H.L., Huang C.W., Huang M.Y., Hou M.F., Juo S.H., Wang J.Y. The functional significance of microRNA-29c in patients with colorectal cancer: A potential circulating biomarker for predicting early relapse. PLoS ONE. 2013;8:e66842. doi: 10.1371/journal.pone.0066842. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

