Generation of Artificial Blastoids Combining miR-200-Mediated Reprogramming and Mechanical Cues
- PMID: 38607067
- PMCID: PMC11011911
- DOI: 10.3390/cells13070628
Generation of Artificial Blastoids Combining miR-200-Mediated Reprogramming and Mechanical Cues
Abstract
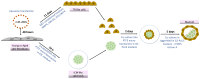
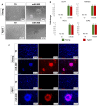
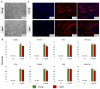
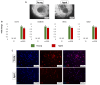
In vitro-generated blastocyst-like structures are of great importance since they recapitulate specific features or processes of early embryogenesis, thus avoiding ethical concerns as well as increasing scalability and accessibility compared to the use of natural embryos. Here, we combine cell reprogramming and mechanical stimuli to create 3D spherical aggregates that are phenotypically similar to those of natural embryos. Specifically, dermal fibroblasts are reprogrammed, exploiting the miR-200 family property to induce a high plasticity state in somatic cells. Subsequently, miR-200-reprogrammed cells are either driven towards the trophectoderm (TR) lineage using an ad hoc induction protocol or encapsulated into polytetrafluoroethylene micro-bioreactors to maintain and promote pluripotency, generating inner cell mass (ICM)-like spheroids. The obtained TR-like cells and ICM-like spheroids are then co-cultured in the same micro-bioreactor and, subsequently, transferred to microwells to encourage blastoid formation. Notably, the above protocol was applied to fibroblasts obtained from young as well as aged donors, with results that highlighted miR-200's ability to successfully reprogram young and aged cells with comparable blastoid rates, regardless of the donor's cell age. Overall, the approach here described represents a novel strategy for the creation of artificial blastoids to be used in the field of assisted reproduction technologies for the study of peri- and early post-implantation mechanisms.
Keywords: ICM-like spheroids; TR-like cells; blastoids; cellular reprogramming; miR-200 family.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Use of miR-200-Mediated Reprogramming and Mechanical Cues to Generate Human Blastocyst Models.Methods Mol Biol. 2025 Aug 5. doi: 10.1007/7651_2025_662. Online ahead of print. Methods Mol Biol. 2025. PMID: 40760313
-
Combination of epigenetic erasing and mechanical cues to generate human epiBlastoids from adult dermal fibroblasts.J Assist Reprod Genet. 2023 May;40(5):1015-1027. doi: 10.1007/s10815-023-02773-4. Epub 2023 Mar 18. J Assist Reprod Genet. 2023. PMID: 36933093 Free PMC article.
-
Use of Epigenetic Cues and Mechanical Stimuli to Generate Blastocyst-Like Structures from Mammalian Skin Dermal Fibroblasts.Methods Mol Biol. 2024;2767:161-173. doi: 10.1007/7651_2023_486. Methods Mol Biol. 2024. PMID: 37199907
-
Modelling human embryogenesis: embryo-like structures spark ethical and policy debate.Hum Reprod Update. 2020 Nov 1;26(6):779-798. doi: 10.1093/humupd/dmaa027. Hum Reprod Update. 2020. PMID: 32712668 Review.
-
An update on human pre- and peri-implantation development: a blueprint for blastoids.Curr Opin Genet Dev. 2023 Dec;83:102125. doi: 10.1016/j.gde.2023.102125. Epub 2023 Oct 4. Curr Opin Genet Dev. 2023. PMID: 37801801 Review.
Cited by
-
Advances in engineered models of peri-gastrulation.iScience. 2025 May 14;28(6):112659. doi: 10.1016/j.isci.2025.112659. eCollection 2025 Jun 20. iScience. 2025. PMID: 40510116 Free PMC article. Review.
-
In Vitro Models of Cardiovascular Disease: Embryoid Bodies, Organoids and Everything in Between.Biomedicines. 2024 Nov 27;12(12):2714. doi: 10.3390/biomedicines12122714. Biomedicines. 2024. PMID: 39767621 Free PMC article. Review.
-
Bioengineering-tissue strategies to model mammalian implantation in vitro.Front Bioeng Biotechnol. 2024 Jul 26;12:1430235. doi: 10.3389/fbioe.2024.1430235. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39132254 Free PMC article.
References
-
- Daoud A.M.P., Popovic M., Dondorp W.J., Bustos M.T., Bredenoord A.L., De Sousa Lopes S.M.C., Van Den Brink S.C., Roelen B.A.J., Guido M.W.R.d.W., Heindryckx B. Modelling Human Embryogenesis: Embryo-like Structures Spark Ethical and Policy Debate. Hum. Reprod. Update. 2020;26:779–798. doi: 10.1093/humupd/dmaa027. - DOI - PubMed

