A Novel Approach for Glioblastoma Treatment by Combining Apoptosis Inducers (TMZ, MTX, and Cytarabine) with E.V.A. (Eltanexor, Venetoclax, and A1210477) Inhibiting XPO1, Bcl-2, and Mcl-1
- PMID: 38607071
- PMCID: PMC11011525
- DOI: 10.3390/cells13070632
A Novel Approach for Glioblastoma Treatment by Combining Apoptosis Inducers (TMZ, MTX, and Cytarabine) with E.V.A. (Eltanexor, Venetoclax, and A1210477) Inhibiting XPO1, Bcl-2, and Mcl-1
Abstract
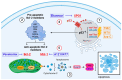
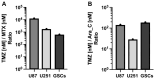
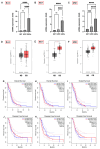
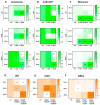
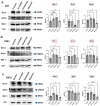
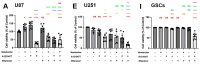
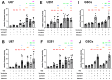
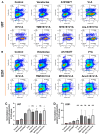
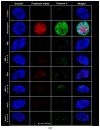
Adjuvant treatment for Glioblastoma Grade 4 with Temozolomide (TMZ) inevitably fails due to therapeutic resistance, necessitating new approaches. Apoptosis induction in GB cells is inefficient, due to an excess of anti-apoptotic XPO1/Bcl-2-family proteins. We assessed TMZ, Methotrexate (MTX), and Cytarabine (Ara-C) (apoptosis inducers) combined with XPO1/Bcl-2/Mcl-1-inhibitors (apoptosis rescue) in GB cell lines and primary GB stem-like cells (GSCs). Using CellTiter-Glo® and Caspase-3 activity assays, we generated dose-response curves and analyzed the gene and protein regulation of anti-apoptotic proteins via PCR and Western blots. Optimal drug combinations were examined for their impact on the cell cycle and apoptosis induction via FACS analysis, paralleled by the assessment of potential toxicity in healthy mouse brain slices. Ara-C and MTX proved to be 150- to 10,000-fold more potent in inducing apoptosis than TMZ. In response to inhibitors Eltanexor (XPO1; E), Venetoclax (Bcl-2; V), and A1210477 (Mcl-1; A), genes encoding for the corresponding proteins were upregulated in a compensatory manner. TMZ, MTX, and Ara-C combined with E, V, and A evidenced highly lethal effects when combined. As no significant cell death induction in mouse brain slices was observed, we conclude that this drug combination is effective in vitro and expected to have low side effects in vivo.
Keywords: Ara-C; Bcl-2; MTX; Mcl-1; SINE; TMZ; XPO1; cytarabine; eltanexor; glioblastoma; methotrexate; temozolomide; venetoclax.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
Treatment of newly diagnosed glioblastoma in the elderly: a network meta-analysis.Cochrane Database Syst Rev. 2020 Mar 23;3(3):CD013261. doi: 10.1002/14651858.CD013261.pub2. Cochrane Database Syst Rev. 2020. PMID: 32202316 Free PMC article.
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
-
The HBP Pathway Inhibitor FR054 Enhances Temozolomide Sensitivity in Glioblastoma Cells by Promoting Ferroptosis and Inhibiting O-GlcNAcylation.CNS Neurosci Ther. 2025 Aug;31(8):e70546. doi: 10.1111/cns.70546. CNS Neurosci Ther. 2025. PMID: 40772310 Free PMC article.
-
The XPO1 Inhibitor Eltanexor Modulates the Wnt/β-Catenin Signaling Pathway to Reduce Colorectal Cancer Tumorigenesis.Cancer Res Commun. 2025 Jul 1;5(7):1140-1154. doi: 10.1158/2767-9764.CRC-25-0052. Cancer Res Commun. 2025. PMID: 40601866 Free PMC article.
Cited by
-
Selinexor as a Therapeutic Target: Advances in Non-small Cell and Small Cell Lung Cancer Treatment Strategies.Recent Pat Anticancer Drug Discov. 2025;20(2):274-284. doi: 10.2174/0115748928322627241016120142. Recent Pat Anticancer Drug Discov. 2025. PMID: 39473203 Review.
-
Sophocarpine inhibits the proliferation and induces apoptosis of glioblastoma cells through regulating the miR-21/PTEN/PI3K/AKT axis.Discov Oncol. 2025 Feb 8;16(1):138. doi: 10.1007/s12672-025-01839-2. Discov Oncol. 2025. PMID: 39921785 Free PMC article.
References
-
- Helseth R., Helseth E., Johannesen T.B., Langberg C.W., Lote K., Rønning P., Scheie D., Vik A., Meling T.R. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol. Scand. 2010;122:159–167. doi: 10.1111/j.1600-0404.2010.01350.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

