GDF-15 Suppresses Puromycin Aminonucleoside-Induced Podocyte Injury by Reducing Endoplasmic Reticulum Stress and Glomerular Inflammation
- PMID: 38607075
- PMCID: PMC11011265
- DOI: 10.3390/cells13070637
GDF-15 Suppresses Puromycin Aminonucleoside-Induced Podocyte Injury by Reducing Endoplasmic Reticulum Stress and Glomerular Inflammation
Abstract
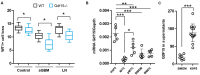
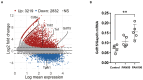
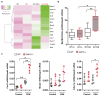
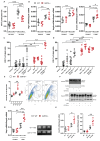
GDF15, also known as MIC1, is a member of the TGF-beta superfamily. Previous studies reported elevated serum levels of GDF15 in patients with kidney disorder, and its association with kidney disease progression, while other studies identified GDF15 to have protective effects. To investigate the potential protective role of GDF15 on podocytes, we first performed in vitro studies using a Gdf15-deficient podocyte cell line. The lack of GDF15 intensified puromycin aminonucleoside (PAN)-triggered endoplasmic reticulum stress and induced cell death in cultivated podocytes. This was evidenced by elevated expressions of Xbp1 and ER-associated chaperones, alongside AnnexinV/PI staining and LDH release. Additionally, we subjected mice to nephrotoxic PAN treatment. Our observations revealed a noteworthy increase in both GDF15 expression and secretion subsequent to PAN administration. Gdf15 knockout mice displayed a moderate loss of WT1+ cells (podocytes) in the glomeruli compared to wild-type controls. However, this finding could not be substantiated through digital evaluation. The parameters of kidney function, including serum BUN, creatinine, and albumin-creatinine ratio (ACR), were increased in Gdf15 knockout mice as compared to wild-type mice upon PAN treatment. This was associated with an increase in the number of glomerular macrophages, neutrophils, inflammatory cytokines, and chemokines in Gdf15-deficient mice. In summary, our findings unveil a novel renoprotective effect of GDF15 during kidney injury and inflammation by promoting podocyte survival and regulating endoplasmic reticulum stress in podocytes, and, subsequently, the infiltration of inflammatory cells via paracrine effects on surrounding glomerular cells.
Keywords: GDF15; endoplasmic reticulum stress; glomerular inflammation; podocytes; podocytopathies.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Glycyrrhizin Alleviates Puromycin Aminonucleoside-Induced Podocyte Injury via Regulating Autophagy and GSDMD-Dependent Pyroptosis.Crit Rev Eukaryot Gene Expr. 2025;35(5):69-80. doi: 10.1615/CritRevEukaryotGeneExpr.2025058852. Crit Rev Eukaryot Gene Expr. 2025. PMID: 40530970
-
Disease-Specific Novel Role of Growth Differentiation Factor 15 in Organ Fibrosis.Int J Mol Sci. 2025 Jun 14;26(12):5713. doi: 10.3390/ijms26125713. Int J Mol Sci. 2025. PMID: 40565178 Free PMC article. Review.
-
The differential expression of MAGI2 in glomerulopathies and its application as a molecular discriminator of podocytopathies.J Transl Med. 2025 Jun 25;23(1):701. doi: 10.1186/s12967-025-06696-9. J Transl Med. 2025. PMID: 40563084 Free PMC article.
-
Nephrin is necessary for podocyte recovery following injury in an adult mature glomerulus.PLoS One. 2018 Jun 20;13(6):e0198013. doi: 10.1371/journal.pone.0198013. eCollection 2018. PLoS One. 2018. PMID: 29924795 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Growth Differentiation Factor-15 as a Potential Biomarker for Renal Involvement in POEMS Syndrome.Int J Nephrol Renovasc Dis. 2025 May 20;18:133-142. doi: 10.2147/IJNRD.S507148. eCollection 2025. Int J Nephrol Renovasc Dis. 2025. PMID: 40416392 Free PMC article.
-
Mechanistic Insights Into Redox Damage of the Podocyte in Hypertension.Hypertension. 2025 Jan;82(1):14-25. doi: 10.1161/HYPERTENSIONAHA.124.22068. Epub 2024 Nov 13. Hypertension. 2025. PMID: 39534957 Review.
References
-
- Inagi R., Nangaku M., Onogi H., Ueyama H., Kitao Y., Nakazato K., Ogawa S., Kurokawa K., Couser W.G., Miyata T. Involvement of Endoplasmic Reticulum (ER) Stress in Podocyte Injury Induced by Excessive Protein Accumulation. Kidney Int. 2005;68:2639–2650. doi: 10.1111/j.1523-1755.2005.00736.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

