Ex Pluribus Unum: The CD4 T Cell Response against Influenza A Virus
- PMID: 38607077
- PMCID: PMC11012043
- DOI: 10.3390/cells13070639
Ex Pluribus Unum: The CD4 T Cell Response against Influenza A Virus
Abstract
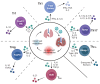
Current Influenza A virus (IAV) vaccines, which primarily aim to generate neutralizing antibodies against the major surface proteins of specific IAV strains predicted to circulate during the annual 'flu' season, are suboptimal and are characterized by relatively low annual vaccine efficacy. One approach to improve protection is for vaccines to also target the priming of virus-specific T cells that can protect against IAV even in the absence of preexisting neutralizing antibodies. CD4 T cells represent a particularly attractive target as they help to promote responses by other innate and adaptive lymphocyte populations and can also directly mediate potent effector functions. Studies in murine models of IAV infection have been instrumental in moving this goal forward. Here, we will review these findings, focusing on distinct subsets of CD4 T cell effectors that have been shown to impact outcomes. This body of work suggests that a major challenge for next-generation vaccines will be to prime a CD4 T cell population with the same spectrum of functional diversity generated by IAV infection. This goal is encapsulated well by the motto 'ex pluribus unum': that an optimal CD4 T cell response comprises many individual specialized subsets responding together.
Keywords: CD4 T cell; Th1; Th17; cytotoxic CD4 T cell; follicular helper cell; influenza virus; regulatory CD4 T cell.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A Bivalent Heterologous DNA Virus-Like-Particle Prime-Boost Vaccine Elicits Broad Protection against both Group 1 and 2 Influenza A Viruses.J Virol. 2017 Apr 13;91(9):e02052-16. doi: 10.1128/JVI.02052-16. Print 2017 May 1. J Virol. 2017. PMID: 28179535 Free PMC article.
-
CCR2 Regulates Vaccine-Induced Mucosal T-Cell Memory to Influenza A Virus.J Virol. 2021 Jul 12;95(15):e0053021. doi: 10.1128/JVI.00530-21. Epub 2021 Jul 12. J Virol. 2021. PMID: 33952647 Free PMC article.
-
Protein Vaccination Directs the CD4+ T Cell Response toward Shared Protective Epitopes That Can Be Recalled after Influenza Virus Infection.J Virol. 2019 Sep 30;93(20):e00947-19. doi: 10.1128/JVI.00947-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31341045 Free PMC article.
-
New Insights into the Generation of CD4 Memory May Shape Future Vaccine Strategies for Influenza.Front Immunol. 2016 Apr 11;7:136. doi: 10.3389/fimmu.2016.00136. eCollection 2016. Front Immunol. 2016. PMID: 27148257 Free PMC article. Review.
-
Modulation of Immune Responses to Influenza A Virus Vaccines by Natural Killer T Cells.Front Immunol. 2020 Oct 20;11:2172. doi: 10.3389/fimmu.2020.02172. eCollection 2020. Front Immunol. 2020. PMID: 33193296 Free PMC article. Review.
Cited by
-
Airway-resident memory CD4 T cell activation accelerates antigen presentation and T cell priming in draining lymph nodes.JCI Insight. 2024 Dec 17;10(3):e182615. doi: 10.1172/jci.insight.182615. JCI Insight. 2024. PMID: 39688906 Free PMC article.
-
Immunological pathogenesis and treatment progress of adenovirus pneumonia in children.Ital J Pediatr. 2025 Jan 9;51(1):4. doi: 10.1186/s13052-024-01836-1. Ital J Pediatr. 2025. PMID: 39789604 Free PMC article. Review.
-
TLR5 activation in respiratory epithelial cells orchestrate mucosal Th17 response through both indirect and direct pathways.Respir Res. 2025 Mar 17;26(1):104. doi: 10.1186/s12931-025-03186-w. Respir Res. 2025. PMID: 40098159 Free PMC article.
-
T4 Phage Displaying Dual Antigen Clusters Against H3N2 Influenza Virus Infection.Vaccines (Basel). 2025 Jan 13;13(1):70. doi: 10.3390/vaccines13010070. Vaccines (Basel). 2025. PMID: 39852849 Free PMC article.
-
Avian influenza mRNA vaccine encoding hemagglutinin provides complete protection against divergent H5N1 viruses in specific-pathogen-free chickens.J Nanobiotechnology. 2025 Jan 29;23(1):55. doi: 10.1186/s12951-025-03156-w. J Nanobiotechnology. 2025. PMID: 39881325 Free PMC article.
References
-
- Powell T.J., Strutt T., Reome J., Hollenbaugh J.A., Roberts A.D., Woodland D.L., Swain S.L., Dutton R.W. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J. Immunol. 2007;178:1030–1038. doi: 10.4049/jimmunol.178.2.1030. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

