Interconnections between the Cation/Alkaline pH-Responsive Slt and the Ambient pH Response of PacC/Pal Pathways in Aspergillus nidulans
- PMID: 38607089
- PMCID: PMC11011638
- DOI: 10.3390/cells13070651
Interconnections between the Cation/Alkaline pH-Responsive Slt and the Ambient pH Response of PacC/Pal Pathways in Aspergillus nidulans
Abstract
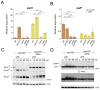
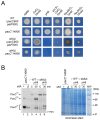
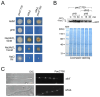
In the filamentous ascomycete Aspergillus nidulans, at least three high hierarchy transcription factors are required for growth at extracellular alkaline pH: SltA, PacC and CrzA. Transcriptomic profiles depending on alkaline pH and SltA function showed that pacC expression might be under SltA regulation. Additional transcriptional studies of PacC and the only pH-regulated pal gene, palF, confirmed both the strong dependence on ambient pH and the function of SltA. The regulation of pacC expression is dependent on the activity of the zinc binuclear (C6) cluster transcription factor PacX. However, we found that the ablation of sltA in the pacX- mutant background specifically prevents the increase in pacC expression levels without affecting PacC protein levels, showing a novel specific function of the PacX factor. The loss of sltA function causes the anomalous proteolytic processing of PacC and a reduction in the post-translational modifications of PalF. At alkaline pH, in a null sltA background, PacC72kDa accumulates, detection of the intermediate PacC53kDa form is extremely low and the final processed form of 27 kDa shows altered electrophoretic mobility. Constitutive ubiquitination of PalF or the presence of alkalinity-mimicking mutations in pacC, such as pacCc14 and pacCc700, resembling PacC53kDa and PacC27kDa, respectively, allowed the normal processing of PacC but did not rescue the alkaline pH-sensitive phenotype caused by the null sltA allele. Overall, data show that Slt and PacC/Pal pathways are interconnected, but the transcription factor SltA is on a higher hierarchical level than PacC on regulating the tolerance to the ambient alkalinity in A. nidulans.
Keywords: abiotic stress; cross regulation; post-translational modifications; signalling; transcriptional factors.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







Similar articles
-
Defining the transcriptional responses of Aspergillus nidulans to cation/alkaline pH stress and the role of the transcription factor SltA.Microb Genom. 2020 Aug;6(8):mgen000415. doi: 10.1099/mgen.0.000415. Epub 2020 Jul 31. Microb Genom. 2020. PMID: 32735212 Free PMC article.
-
Refining the pH response in Aspergillus nidulans: a modulatory triad involving PacX, a novel zinc binuclear cluster protein.Mol Microbiol. 2015 Dec;98(6):1051-72. doi: 10.1111/mmi.13173. Epub 2015 Oct 16. Mol Microbiol. 2015. PMID: 26303777 Free PMC article.
-
A second component of the SltA-dependent cation tolerance pathway in Aspergillus nidulans.Fungal Genet Biol. 2015 Sep;82:116-28. doi: 10.1016/j.fgb.2015.06.002. Epub 2015 Jun 26. Fungal Genet Biol. 2015. PMID: 26119498 Free PMC article.
-
Function of pH-dependent transcription factor PacC in regulating development, pathogenicity, and mycotoxin biosynthesis of phytopathogenic fungi.FEBS J. 2022 Apr;289(7):1723-1730. doi: 10.1111/febs.15808. Epub 2021 Mar 26. FEBS J. 2022. PMID: 33751796 Review.
-
pH regulation of gene expression in fungi.Fungal Genet Biol. 2000 Mar;29(2):61-71. doi: 10.1006/fgbi.2000.1188. Fungal Genet Biol. 2000. PMID: 10919375 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

