Three-Dimensional-Printed GelMA-KerMA Composite Patches as an Innovative Platform for Potential Tissue Engineering of Tympanic Membrane Perforations
- PMID: 38607098
- PMCID: PMC11013928
- DOI: 10.3390/nano14070563
Three-Dimensional-Printed GelMA-KerMA Composite Patches as an Innovative Platform for Potential Tissue Engineering of Tympanic Membrane Perforations
Abstract
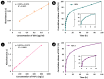
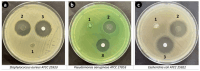
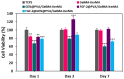
Tympanic membrane (TM) perforations, primarily induced by middle ear infections, the introduction of foreign objects into the ear, and acoustic trauma, lead to hearing abnormalities and ear infections. We describe the design and fabrication of a novel composite patch containing photocrosslinkable gelatin methacryloyl (GelMA) and keratin methacryloyl (KerMA) hydrogels. GelMA-KerMA patches containing conical microneedles in their design were developed using the digital light processing (DLP) 3D printing approach. Following this, the patches were biofunctionalized by applying a coaxial coating with PVA nanoparticles loaded with gentamicin (GEN) and fibroblast growth factor (FGF-2) with the Electrohydrodynamic Atomization (EHDA) method. The developed nanoparticle-coated 3D-printed patches were evaluated in terms of their chemical, morphological, mechanical, swelling, and degradation behavior. In addition, the GEN and FGF-2 release profiles, antimicrobial properties, and biocompatibility of the patches were examined in vitro. The morphological assessment verified the successful fabrication and nanoparticle coating of the 3D-printed GelMA-KerMA patches. The outcomes of antibacterial tests demonstrated that GEN@PVA/GelMA-KerMA patches exhibited substantial antibacterial efficacy against Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli. Furthermore, cell culture studies revealed that GelMA-KerMA patches were biocompatible with human adipose-derived mesenchymal stem cells (hADMSC) and supported cell attachment and proliferation without any cytotoxicity. These findings indicated that biofunctional 3D-printed GelMA-KerMA patches have the potential to be a promising therapeutic approach for addressing TM perforations.
Keywords: DLP 3D printing; FGF-2; gelatin methacryloyl; gentamicin; keratin methacrylolyl; nanoparticle; tympanic membrane perforation.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Fabrication of tissue-engineered tympanic membrane patches using 3D-Printing technology.J Mech Behav Biomed Mater. 2021 Feb;114:104219. doi: 10.1016/j.jmbbm.2020.104219. Epub 2020 Dec 2. J Mech Behav Biomed Mater. 2021. PMID: 33302170
-
A self-healing hydrogel and injectable cryogel of gelatin methacryloyl-polyurethane double network for 3D printing.Acta Biomater. 2023 Jul 1;164:124-138. doi: 10.1016/j.actbio.2023.04.023. Epub 2023 Apr 22. Acta Biomater. 2023. PMID: 37088162
-
3D-Bioprinted Gelatin Methacryloyl-Strontium-Doped Hydroxyapatite Composite Hydrogels Scaffolds for Bone Tissue Regeneration.Polymers (Basel). 2024 Jul 6;16(13):1932. doi: 10.3390/polym16131932. Polymers (Basel). 2024. PMID: 39000787 Free PMC article.
-
Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering.J Biomed Mater Res A. 2022 Mar;110(3):708-724. doi: 10.1002/jbm.a.37310. Epub 2021 Sep 24. J Biomed Mater Res A. 2022. PMID: 34558808 Review.
-
Recent advances on gelatin methacrylate hydrogels with controlled microstructures for tissue engineering.Int J Biol Macromol. 2022 Nov 30;221:91-107. doi: 10.1016/j.ijbiomac.2022.08.171. Epub 2022 Aug 31. Int J Biol Macromol. 2022. PMID: 36057299 Review.
Cited by
-
The Convergence of Nanotechnology and Biotechnology in Modern Medicine.Nanomaterials (Basel). 2025 Jan 24;15(3):182. doi: 10.3390/nano15030182. Nanomaterials (Basel). 2025. PMID: 39940158 Free PMC article.
-
Tough and self-adhesive zwitterionic hydrogels with mechano-responsive release of bFGF for tympanic membrane repair.Mater Today Bio. 2024 Aug 24;28:101212. doi: 10.1016/j.mtbio.2024.101212. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39687063 Free PMC article.
-
Manufacturing Radially Aligned PCL Nanofibers Reinforced With Sulfated Levan and Evaluation of its Biological Activity for Healing Tympanic Membrane Perforations.Macromol Biosci. 2025 Jan;25(1):e2400291. doi: 10.1002/mabi.202400291. Epub 2024 Oct 26. Macromol Biosci. 2025. PMID: 39461894 Free PMC article.
-
Advances in 3D printing for the repair of tympanic membrane perforation: a comprehensive review.Front Bioeng Biotechnol. 2024 Aug 12;12:1439499. doi: 10.3389/fbioe.2024.1439499. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39188376 Free PMC article. Review.
-
Development of Graphene Oxide-Based Anticancer Drug Combination Functionalized with Folic Acid as Nanocarrier for Targeted Delivery of Methotrexate.Pharmaceutics. 2024 Jun 20;16(6):837. doi: 10.3390/pharmaceutics16060837. Pharmaceutics. 2024. PMID: 38931957 Free PMC article.
References
-
- Anand S., Danti S., Moroni L., Mota C. Regenerative Therapies for Tympanic Membrane. Prog. Mater. Sci. 2022;127:100942. doi: 10.1016/j.pmatsci.2022.100942. - DOI
-
- Rathnakara S.H., Raju S.R., Kumar R., Hegde G. Silk Fibroin Nanofiber Scaffold (SFNS) as a Graft Material for Myringoplasty—In Vitro and In Vivo. Materialia. 2022;26:101562. doi: 10.1016/j.mtla.2022.101562. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

