Strontium and Zinc Co-Doped Mesoporous Bioactive Glass Nanoparticles for Potential Use in Bone Tissue Engineering Applications
- PMID: 38607110
- PMCID: PMC11013354
- DOI: 10.3390/nano14070575
Strontium and Zinc Co-Doped Mesoporous Bioactive Glass Nanoparticles for Potential Use in Bone Tissue Engineering Applications
Abstract
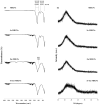
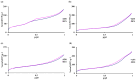
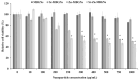
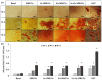
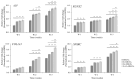
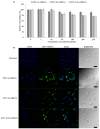
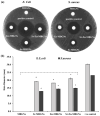
Mesoporous bioactive glass nanoparticles (MBGNs) have attracted significant attention as multifunctional nanocarriers for various applications in both hard and soft tissue engineering. In this study, multifunctional strontium (Sr)- and zinc (Zn)-containing MBGNs were successfully synthesized via the microemulsion-assisted sol-gel method combined with a cationic surfactant (cetyltrimethylammonium bromide, CTAB). Sr-MBGNs, Zn-MBGNs, and Sr-Zn-MBGNs exhibited spherical shapes in the nanoscale range of 100 ± 20 nm with a mesoporous structure. Sr and Zn were co-substituted in MBGNs (60SiO2-40CaO) to induce osteogenic potential and antibacterial properties without altering their size, morphology, negative surface charge, amorphous nature, mesoporous structure, and pore size. The synthesized MBGNs facilitated bioactivity by promoting the formation of an apatite-like layer on the surface of the particles after immersion in Simulated Body Fluid (SBF). The effect of the particles on the metabolic activity of human mesenchymal stem cells was concentration-dependent. The hMSCs exposed to Sr-MBGNs, Zn-MBGNs, and Sr-Zn-MBGNs at 200 μg/mL enhanced calcium deposition and osteogenic differentiation without osteogenic supplements. Moreover, the cellular uptake and internalization of Sr-MBGNs, Zn-MBGNs, and Sr-Zn-MBGNs in hMSCs were observed. These novel particles, which exhibited multiple functionalities, including promoting bone regeneration, delivering therapeutic ions intracellularly, and inhibiting the growth of Staphylococcus aureus and Escherichia coli, are potential nanocarriers for bone regeneration applications.
Keywords: antibacterial activity; bioactive glass nanoparticle; bioactivity; intracellular delivery; mesoporous.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Enhanced functionalities of biomaterials through metal ion surface modification.Front Bioeng Biotechnol. 2025 Apr 14;13:1522442. doi: 10.3389/fbioe.2025.1522442. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40297280 Free PMC article. Review.
-
Multifunctional zinc ion doped sol - gel derived mesoporous bioactive glass nanoparticles for biomedical applications.Bioact Mater. 2019 Oct 27;4:312-321. doi: 10.1016/j.bioactmat.2019.10.002. eCollection 2019 Dec. Bioact Mater. 2019. PMID: 31709314 Free PMC article.
-
Synthesis and Characterization of Silver-Strontium (Ag-Sr)-Doped Mesoporous Bioactive Glass Nanoparticles.Gels. 2021 Mar 24;7(2):34. doi: 10.3390/gels7020034. Gels. 2021. PMID: 33805013 Free PMC article.
-
Multifunctional Sr,Mg-Doped Mesoporous Bioactive Glass Nanoparticles for Simultaneous Bone Regeneration and Drug Delivery.Int J Mol Sci. 2024 Jul 24;25(15):8066. doi: 10.3390/ijms25158066. Int J Mol Sci. 2024. PMID: 39125634 Free PMC article.
-
Advanced applications of strontium-containing biomaterials in bone tissue engineering.Mater Today Bio. 2023 Apr 15;20:100636. doi: 10.1016/j.mtbio.2023.100636. eCollection 2023 Jun. Mater Today Bio. 2023. PMID: 37441138 Free PMC article. Review.
Cited by
-
Advances in Zinc-Containing Bioactive Glasses: A Comprehensive Review.J Funct Biomater. 2024 Sep 8;15(9):258. doi: 10.3390/jfb15090258. J Funct Biomater. 2024. PMID: 39330233 Free PMC article. Review.
-
Enhanced functionalities of biomaterials through metal ion surface modification.Front Bioeng Biotechnol. 2025 Apr 14;13:1522442. doi: 10.3389/fbioe.2025.1522442. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40297280 Free PMC article. Review.
-
Strategic incorporation of metal ions in bone regenerative scaffolds: multifunctional platforms for advancing osteogenesis.Regen Biomater. 2025 Jul 2;12:rbaf068. doi: 10.1093/rb/rbaf068. eCollection 2025. Regen Biomater. 2025. PMID: 40755870 Free PMC article. Review.
References
-
- Bari A., Bloise N., Fiorilli S., Novajra G., Vallet-Regí M., Bruni G., Torres-Pardo A., González-Calbet J.M., Visai L., Vitale-Brovarone C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017;55:493–504. doi: 10.1016/j.actbio.2017.04.012. - DOI - PubMed
-
- Parvizi J., Kim G.K. Chapter 32—Bone Grafting. In: Parvizi J., Kim G.K., editors. High Yield Orthopaedics. W.B. Saunders; Philadelphia, PA, USA: 2010. pp. 64–65.
Grants and funding
- RGNS 64-098/Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innova-tion(OPS MHESI), Thailand Science Research and Innovation (TSRI)
- FRB660073/0164/Thailand Science Research and Innovation (TSRI), Basic Research Fund: Fiscal year 2023
- 27149/NTUT-KMUTT Joint Research Program, King Mongkut's University of Technology Thonburi
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

