Exploratory mass cytometry analysis reveals immunophenotypes of cancer treatment-related pneumonitis
- PMID: 38607373
- PMCID: PMC11014725
- DOI: 10.7554/eLife.87288
Exploratory mass cytometry analysis reveals immunophenotypes of cancer treatment-related pneumonitis
Abstract
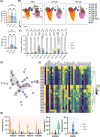
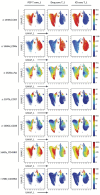
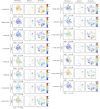
Anticancer treatments can result in various adverse effects, including infections due to immune suppression/dysregulation and drug-induced toxicity in the lung. One of the major opportunistic infections is Pneumocystis jirovecii pneumonia (PCP), which can cause severe respiratory complications and high mortality rates. Cytotoxic drugs and immune-checkpoint inhibitors (ICIs) can induce interstitial lung diseases (ILDs). Nonetheless, the differentiation of these diseases can be difficult, and the pathogenic mechanisms of such diseases are not yet fully understood. To better comprehend the immunophenotypes, we conducted an exploratory mass cytometry analysis of immune cell subsets in bronchoalveolar lavage fluid from patients with PCP, cytotoxic drug-induced ILD (DI-ILD), and ICI-associated ILD (ICI-ILD) using two panels containing 64 markers. In PCP, we observed an expansion of the CD16+ T cell population, with the highest CD16+ T proportion in a fatal case. In ICI-ILD, we found an increase in CD57+ CD8+ T cells expressing immune checkpoints (TIGIT+ LAG3+ TIM-3+ PD-1+), FCRL5+ B cells, and CCR2+ CCR5+ CD14+ monocytes. These findings uncover the diverse immunophenotypes and possible pathomechanisms of cancer treatment-related pneumonitis.
Keywords: Pneumocystis jirovecii pneumonia; bronchoalveolar lavage fluid; human; immune-checkpoint inhibitor; immunology; inflammation; mass cytometry.
© 2023, Yanagihara, Hata et al.
Conflict of interest statement
TY This research was supported by the Kakihara Foundation and Boehringer Ingelheim, KH, KM, KK, KS, KT, SI, YB, YF, IO No competing interests declared
Figures







Update of
- doi: 10.1101/2023.02.21.529383
- doi: 10.7554/eLife.87288.1
- doi: 10.7554/eLife.87288.2
- doi: 10.7554/eLife.87288.3
References
-
- Axelrod ML, Meijers WC, Screever EM, Qin J, Carroll MG, Sun X, Tannous E, Zhang Y, Sugiura A, Taylor BC, Hanna A, Zhang S, Amancherla K, Tai W, Wright JJ, Wei SC, Opalenik SR, Toren AL, Rathmell JC, Ferrell PB, Phillips EJ, Mallal S, Johnson DB, Allison JP, Moslehi JJ, Balko JM. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature. 2022;611:818–826. doi: 10.1038/s41586-022-05432-3. - DOI - PMC - PubMed
-
- Björkström NK, Gonzalez VD, Malmberg K-J, Falconer K, Alaeus A, Nowak G, Jorns C, Ericzon B-G, Weiland O, Sandberg JK, Ljunggren H-G. Elevated numbers of Fc gamma RIIIA+ (CD16+) effector CD8 T cells with NK cell-like function in chronic hepatitis C virus infection. Journal of Immunology. 2008;181:4219–4228. doi: 10.4049/jimmunol.181.6.4219. - DOI - PubMed
MeSH terms
Associated data
- Dryad/10.5061/dryad.2ngf1vhxd
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

