Postinfusion PD-1+ CD8+ CAR T cells identify patients responsive to CD19 CAR T-cell therapy in non-Hodgkin lymphoma
- PMID: 38607381
- PMCID: PMC11222947
- DOI: 10.1182/bloodadvances.2023012073
Postinfusion PD-1+ CD8+ CAR T cells identify patients responsive to CD19 CAR T-cell therapy in non-Hodgkin lymphoma
Abstract
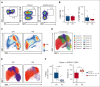
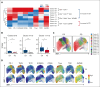
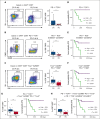
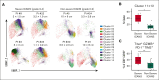
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized treatment for relapsed/refractory B-cell non-Hodgkin lymphoma (NHL). Robust biomarkers and a complete understanding of CAR T-cell function in the postinfusion phase remain limited. Here, we used a 37-color spectral flow cytometry panel to perform high dimensional single-cell analysis of postinfusion samples in 26 patients treated with CD28 costimulatory domain containing commercial CAR T cells for NHL and focused on computationally gated CD8+ CAR T cells. We found that the presence of postinfusion Programmed cell death protein 1 (PD-1)+ CD8+ CAR T cells at the day 14 time point highly correlated with the ability to achieve complete response (CR) by 6 months. Further analysis identified multiple subtypes of CD8+ PD-1+ CAR T cells, including PD-1+ T cell factor 1 (TCF1)+ stem-like CAR T cells and PD-1+ T-cell immunoglobulin and mucin-domain containing-3 (TIM3)+ effector-like CAR T cells that correlated with improved clinical outcomes such as response and progression-free survival. Additionally, we identified a subset of PD-1+ CD8+ CAR+ T cells with effector-like function that was increased in patients who achieved a CR and was associated with grade 3 or higher immune effector cell-associated neurotoxicity syndrome. Here, we identified robust biomarkers of response to CD28 CAR T cells and highlight the importance of PD-1 positivity in CD8+ CAR T cells after infusion in achieving CR.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosures: T.J.V receives research funding from MorphoSys, Incyte, Genmab, AbbVie, Recordati, Viracta Therapeutics, and AstraZeneca; and has consulted for Novartis and Recordati. A.S.K. receives research funding from AstraZeneca and BeiGene; and consults for AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb (BMS), Kite, a Gilead Company, Janssen, and Loxo@Lilly. D.A.B. receives research funding from Novartis, Nurix Therapeutics, Kite, a Gilead Company, and Incyte; and has consulted for Novartis, Nurix Therapeutics, ADC Therapeutics, and Kite, a Gilead Company. Y.S. has received research funding from BMS, Celgene, TG Therapeutics, BeiGene, AbbVie, and Genmab. J.C.R receives research funding from Merck, Corvus Pharmaceuticals, and Kymera Therapeutics; and consults for Acrotech Biopharma, and Kyowa Kirin. W.H. receives research funding from Incyte. B.C. received research funding from Genentech, Acerta, Millenium, and BMS. S.J. received research funding from Kite, a Gilead Company and BMS; and serves on advisory boards for Kite, a Gilead Company, BMS, Caribou Biosciences, and CRISPR Therapeutics. The remaining authors declare no competing financial interests.
Figures


References
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
-
- Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

