Association of leukemic molecular profile with efficacy of inotuzumab ozogamicin in adults with relapsed/refractory ALL
- PMID: 38607410
- PMCID: PMC11225676
- DOI: 10.1182/bloodadvances.2023012430
Association of leukemic molecular profile with efficacy of inotuzumab ozogamicin in adults with relapsed/refractory ALL
Abstract
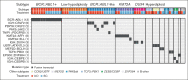
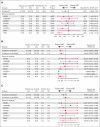
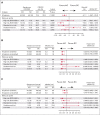
The phase 3 INO-VATE trial demonstrated higher rates of remission, measurable residual disease negativity, and improved overall survival for patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL) who received inotuzumab ozogamicin (InO) vs standard-of-care chemotherapy (SC). Here, we examined associations between genomic alterations and the efficacy of InO. Of 326 randomized patients, 91 (InO, n = 43; SC, n = 48) had samples evaluable for genomic analysis. The spectrum of gene fusions and other genomic alterations observed was comparable with prior studies of adult ALL. Responses to InO were observed in all leukemic subtypes, genomic alterations, and risk groups. Significantly higher rates of complete remission (CR)/CR with incomplete count recovery were observed with InO vs SC in patients with BCR::ABL1-like ALL (85.7% [6/7] vs 0% [0/5]; P = .0076), with TP53 alterations (100% [5/5] vs 12.5% [1/8]; P = .0047), and in the high-risk BCR::ABL1- (BCR::ABL1-like, low-hypodiploid, KMT2A-rearranged) group (83.3% [10/12] vs 10.5% [2/19]; P < .0001). This retrospective, exploratory analysis of the INO-VATE trial demonstrated potential for benefit with InO for patients with R/R ALL across leukemic subtypes, including BCR::ABL1-like ALL, and for those bearing diverse genomic alterations. Further confirmation of the efficacy of InO in patients with R/R ALL exhibiting the BCR::ABL1-like subtype or harboring TP53 alterations is warranted. This trial was registered at www.ClinicalTrials.gov as #NCT01564784.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: H.K. received honoraria from AbbVie, Amgen, Daiichi-Sankyo, Pfizer, Bio Ascend, Novartis, Takeda, Adaptive Biotechnologies, Aptitude Health, Delta-Fly, Janssen Global, and Oxford Biomedical; research support from AbbVie, Amgen, Daiichi-Sankyo, and Pfizer; grants from Ascentage, Bristol Myers Squibb, Immunogen, Jazz Pharmaceuticals, and Sanofi; and serves on the advisory board of Actinium. D.J.D. received honoraria from Amgen, Autolus, Agios, Blueprint Pharmaceuticals, Forty Seven, Incyte, Jazz Pharmaceuticals, Kite Pharma, Novartis, Pfizer, Shire, and Takeda, and research support from AbbVie, GlycoMimetics, Novartis, and Blueprint Medicines. M.S. received research support and honoraria from Pfizer. M.L. received research support and honoraria from Pfizer and Amgen. W.S. received research support and/or honoraria from ADC Therapeutics, Agios, Amgen, Astellas, Gilead, Jazz, Kite, Pfizer, and Sigma-Tau. N.G. received research support and honoraria from Pfizer and Amgen. S.O. received research support and honoraria from Pfizer. E.J. received research support and consultancy fees from Amgen, Pfizer, Takeda, Adaptive Biotechnologies, AbbVie, Bristol Myers Squibb, Spectrum, and Genentech. R.D.C. reports consultancy for Amgen, Jazz, Kite/Gilead, and Pfizer; serves on boards and committees of PeproMene Bio and Autolus; received research support from Amgen, Kite/Gilead, Pfizer, Servier, and Vanda Pharmaceuticals; received honoraria from Kite/Gilead; and reports spouse employed by, and owns stocks in, Seagen. X.L. is a previous employee of Pfizer and is now an employee of Mirati Therapeutics Inc. C.G.M. reports research support from Pfizer and AbbVie; received honoraria from Amgen and Illumina; and reports equity in Amgen. A.A. reports consulting for Jazz Pharmaceuticals, Beam Theraputics, GlycoMimetics, Amgen, Pfizer, and Novartis, and received research support from Seattle Genetics, Immunogen, Glycomimetics, AbbVie, Pfizer, Amgen, Kite, and MacroGenics. A.D.L., R.Y., and E.V. are employees of, and own stock in, Pfizer. The remaining authors declare no competing financial interests.
Figures



Similar articles
-
Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study.Cancer. 2019 Jul 15;125(14):2474-2487. doi: 10.1002/cncr.32116. Epub 2019 Mar 28. Cancer. 2019. PMID: 30920645 Free PMC article. Clinical Trial.
-
Inotuzumab Ozogamicin for Relapsed/Refractory Acute Lymphoblastic Leukemia in the INO-VATE Trial: CD22 Pharmacodynamics, Efficacy, and Safety by Baseline CD22.Clin Cancer Res. 2021 May 15;27(10):2742-2754. doi: 10.1158/1078-0432.CCR-20-2399. Epub 2021 Feb 18. Clin Cancer Res. 2021. PMID: 33602684
-
Impact of minimal residual disease status in patients with relapsed/refractory acute lymphoblastic leukemia treated with inotuzumab ozogamicin in the phase III INO-VATE trial.Leuk Res. 2020 Jan;88:106283. doi: 10.1016/j.leukres.2019.106283. Epub 2019 Nov 25. Leuk Res. 2020. PMID: 31790983 Clinical Trial.
-
Characterizing the ideal patient for treatment with inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia: a systematic literature review.Expert Rev Hematol. 2025 Jan;18(1):91-103. doi: 10.1080/17474086.2025.2450223. Epub 2025 Jan 14. Expert Rev Hematol. 2025. PMID: 39778191
-
Inotuzumab: from preclinical development to success in B-cell acute lymphoblastic leukemia.Blood Adv. 2019 Jan 8;3(1):96-104. doi: 10.1182/bloodadvances.2018026211. Blood Adv. 2019. PMID: 30622147 Free PMC article. Review.
Cited by
-
Antibody-Based and Other Novel Agents in Adult B-Cell Acute Lymphoblastic Leukemia.Cancers (Basel). 2025 Feb 25;17(5):779. doi: 10.3390/cancers17050779. Cancers (Basel). 2025. PMID: 40075627 Free PMC article. Review.
-
Therapeutic Strategies for Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia in Adult Patients: Optimizing the Use of Monoclonal Antibodies.Eur J Haematol. 2025 Jun;114(6):938-952. doi: 10.1111/ejh.14405. Epub 2025 Mar 6. Eur J Haematol. 2025. PMID: 40045912 Free PMC article. Review.
References
-
- DiJoseph JF, Armellino DC, Boghaert ER, et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103(5):1807–1814. - PubMed
-
- Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13(4):403–411. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

