Enzalutamide: Understanding and Managing Drug Interactions to Improve Patient Safety and Drug Efficacy
- PMID: 38607520
- PMCID: PMC11182822
- DOI: 10.1007/s40264-024-01415-7
Enzalutamide: Understanding and Managing Drug Interactions to Improve Patient Safety and Drug Efficacy
Abstract
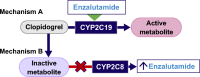
Enzalutamide is an oral androgen receptor signaling inhibitor utilized in the treatment of men with prostate cancer. It is a moderate inducer of the cytochrome P450 (CYP) enzymes CYP2C9 and CYP2C19, and a strong inducer of CYP3A4. It was also shown to be a mild inhibitor of the efflux transporter P-glycoprotein in patients with prostate cancer. Enzalutamide is primarily metabolized by CYP3A4 and CYP2C8. The risk of enzalutamide drug interactions arises primarily when it is coadministered with other drugs that interact with these CYPs, including CYP3A4. In this review, we begin by providing an overview of enzalutamide including its dosing, use in special populations, pharmacokinetics, changes to its prescribing information, and potential for interaction with coadministered drugs. Enzalutamide interactions with drugs from a wide range of medication classes commonly prescribed to patients with prostate cancer are described, including oral androgen deprivation therapy, agents used to treat a range of cardiovascular diseases, antidiabetic drugs, antidepressants, anti-seizure medications, common urology medications, analgesics, proton pump inhibitors, immunosuppressants, and antigout drugs. Enzalutamide interactions with common vitamins and supplements are also briefly discussed. This review provides a resource for healthcare practitioners and patients that will help provide a basis for the understanding and management of enzalutamide drug-drug interactions to inform decision making, improve patient safety, and optimize drug efficacy.
Plain language summary
Enzalutamide is a drug that is used to treat various stages of advanced prostate cancer, a type of cancer that begins in the prostate and may spread beyond the prostate. Enzalutamide stops testosterone from stimulating prostate cancer growth. Like other drugs, enzalutamide enters the bloodstream, and then is processed and removed from the body. Sometimes, when a person takes multiple drugs, one drug can make it difficult for the body to process and remove one or more of the other drugs. This is referred to as a drug interaction. Enzalutamide drug interactions can cause the level of other drugs in the body to increase or decrease in an abnormal way. It is also possible for certain other drugs to alter the levels of enzalutamide. Drug interactions that cause the level of a drug to get too low can prevent that drug from working effectively, whereas drug interactions that cause the level of a drug to get too high can lead to side effects of that drug. People with prostate cancer are mostly aged 65 years or older and often take medications to treat a variety of diseases. Examples include medications to treat heart conditions, diabetes, high cholesterol, high blood pressure, and many other conditions. Here, we describe enzalutamide drug interactions with these types of medications. Our goal is to provide a resource to help healthcare providers and patients better understand enzalutamide drug interactions and how to manage them to improve patient safety and drug effectiveness.
© 2024. Pfizer Inc.
Conflict of interest statement
Brandon Lennep has received consulting fees from Pfizer Inc. Jesse Mack is an employee of Astellas Pharma Inc. and was a consultant for Pfizer prior to joining Astellas Pharma Inc. Srinivasu Poondru is an employee of Astellas Pharma Inc. Brooke Looney receives research support from AstraZeneca Inc. and Pfizer Inc. and has served as a speaker for AstraZeneca Inc. Judeth Bianco is an employee of Pfizer Inc. Alicia Morgans has received consulting fees from Astellas Pharma Inc., AAA Pharma Inc., AstraZeneca Pharma LP, Bayer HealthCare Pharma LLC, Dendreon Pharma LLC, Janssen Pharma LLC, Exelixis Inc., Myovant Sciences, Inc., Merck & Co. Inc., Novartis AG, Lantheus, Telix Pharma Limited, Sanofi S.A., and Pfizer Inc., research fees from Bayer HealthCare Pharma LLC, Myovant Sciences Inc., and Pfizer Inc., and travel support from Telix Pharma Limited and Sanofi S.A. Elizabeth Hood and Monique Williams have no financial interests to declare.
Figures


Similar articles
-
A drug safety evaluation of enzalutamide to treat advanced prostate cancer.Expert Opin Drug Saf. 2021 Jul;20(7):741-749. doi: 10.1080/14740338.2021.1919620. Epub 2021 Jun 11. Expert Opin Drug Saf. 2021. PMID: 34114527 Review.
-
Pharmacokinetic Drug Interaction Studies with Enzalutamide.Clin Pharmacokinet. 2015 Oct;54(10):1057-69. doi: 10.1007/s40262-015-0283-1. Clin Pharmacokinet. 2015. PMID: 25929560 Free PMC article. Clinical Trial.
-
The role of drug-drug interactions in prostate cancer treatment: Focus on abiraterone acetate/prednisone and enzalutamide.Cancer Treat Rev. 2017 Apr;55:71-82. doi: 10.1016/j.ctrv.2017.03.001. Epub 2017 Mar 9. Cancer Treat Rev. 2017. PMID: 28340451 Review.
-
Enzalutamide With Radiation Therapy for Intermediate-Risk Prostate Cancer: A Phase 2 Study.Int J Radiat Oncol Biol Phys. 2021 Aug 1;110(5):1416-1422. doi: 10.1016/j.ijrobp.2021.02.027. Epub 2021 Feb 23. Int J Radiat Oncol Biol Phys. 2021. PMID: 33636278 Clinical Trial.
-
Treatment of non-metastatic castration-resistant prostate cancer: facing age-related comorbidities and drug-drug interactions.Expert Opin Drug Metab Toxicol. 2022 Sep;18(9):601-613. doi: 10.1080/17425255.2022.2122812. Epub 2022 Sep 16. Expert Opin Drug Metab Toxicol. 2022. PMID: 36111393 Review.
Cited by
-
Integrated bioinformatics analysis reveals that OPRK1 inhibits ferroptosis and induces enzalutamide resistance in prostate cancer.Eur J Med Res. 2025 Apr 15;30(1):279. doi: 10.1186/s40001-025-02484-9. Eur J Med Res. 2025. PMID: 40229787 Free PMC article.
-
Packing the Punch: Current and Emerging Treatment Strategies in Metastatic Castration-Sensitive Prostate Cancer.Curr Urol Rep. 2025 Jun 21;26(1):50. doi: 10.1007/s11934-025-01272-6. Curr Urol Rep. 2025. PMID: 40542935 Free PMC article. Review.
References
-
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer 2022. Available from: https://seer.cancer.gov/statfacts/html/prost.html. Accessed 10 Apr 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

