First-line pembrolizumab plus chemotherapy for advanced/metastatic esophageal cancer: 1-year extended follow-up in the Japanese subgroup of the phase 3 KEYNOTE-590 study
- PMID: 38607538
- PMCID: PMC11199245
- DOI: 10.1007/s10388-024-01053-z
First-line pembrolizumab plus chemotherapy for advanced/metastatic esophageal cancer: 1-year extended follow-up in the Japanese subgroup of the phase 3 KEYNOTE-590 study
Abstract
Background: First-line pembrolizumab plus chemotherapy (pembrolizumab-chemotherapy) demonstrated improved efficacy and a manageable safety profile versus placebo plus chemotherapy (placebo-chemotherapy) in the subgroup analysis of Japanese patients with advanced/metastatic esophageal cancer in KEYNOTE-590 at a median follow-up of 24.4 months. Longer-term data from the Japanese subgroup analysis of KEYNOTE-590 are reported.
Methods: Patients were randomly assigned 1:1 to pembrolizumab 200 mg or placebo every 3 weeks for ≤ 35 cycles plus chemotherapy (cisplatin 80 mg/m2 and 5-fluorouracil 800 mg/m2/day). Endpoints included overall survival (OS) and progression-free survival (PFS; investigator-assessed per RECIST v1.1; dual primary) and safety (secondary). Early tumor shrinkage (ETS) and depth of response (DpR) were assessed post hoc.
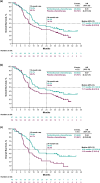
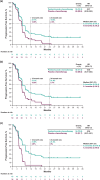
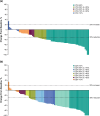
Results: Overall, 141 patients were enrolled in Japan. As of July 9, 2021, median follow-up was 36.6 months (range, 29.8-45.7). Pembrolizumab-chemotherapy showed a trend toward favorable OS (hazard ratio [HR], 0.70; 95% confidence interval [CI] 0.47-1.03) and PFS (0.57; 0.39-0.83) versus placebo-chemotherapy. In the pembrolizumab-chemotherapy group, patients with ETS ≥ 20% (55/74; 74.3%) versus < 20% (19/74; 25.7%) had favorable OS (HR, 0.23; 95% CI 0.12-0.42) and PFS (0.24; 0.13-0.43). Patients with DpR ≥ 60% (31/74; 41.9%) versus < 60% (43/74; 58.1%) had favorable OS (HR, 0.37; 95% CI 0.20-0.68) and PFS (0.24; 0.13-0.43). Grade 3-5 treatment-related adverse events occurred in 55/74 patients (74.3%) with pembrolizumab-chemotherapy and 41/67 patients (61.2%) with placebo-chemotherapy.
Conclusions: With longer-term follow-up of Japanese patients with advanced/metastatic esophageal cancer, efficacy continued to favor pembrolizumab-chemotherapy compared with placebo-chemotherapy, with no new safety signals observed.
Clinical trial registration: ClinicalTrials.gov, NCT03189719.
Keywords: Esophageal squamous cell carcinoma; Immune checkpoint inhibitors; Immunotherapy; Pembrolizumab; Programmed cell death ligand 1.
© 2024. The Author(s).
Conflict of interest statement
Ken Kato reports research funds to his institution from Merck & Co., Inc., during the conduct of the present study; research funds/grants paid to his institution from ONO Pharmaceutical Co., Ltd., Bristol Myers Squibb, Taiho Pharmaceutical, AstraZeneca, Janssen, and BeiGene; honoraria from ONO Pharmaceutical, Bristol Myers Squibb, and Taiho Pharmaceutical; and consultation fees from Chugai Pharmaceutical Co., Ltd., and Daiichi Sankyo, outside the submitted work. Takashi Kojima reports research grant to his institution from ONO Pharmaceutical Co., Ltd., MSD, Amgen Astellas BioPharma, Taiho Pharmaceutical, Shionogi, BeiGene Ltd., AstraZeneca, Chugai Pharmaceutical Co., Ltd., and Parexel International; honoraria from MSD, Oncolys BioPharma, Astellas Pharma, Bristol Myers Squibb, Boehringer Ingelheim, Kyowa Kirin, EA Pharma, Covidien Japan, 3H Clinical Trial; and personal fees for membership of advisory board from ONO Pharmaceutical Co., Ltd., Taiho Pharmaceutical, Japanese Society of Pharmaceutical Health Care and Sciences, Boehringer Ingelheim, and LiangYiHui Healthcare | Oncology News China, outside the submitted work. Hiroki Hara reports research support from MSD for the present study; research funding to his institution from ALX Oncology, Amgen, AstraZeneca, Astellas Pharma, Bayel, BeiGene, Boehringer Ingelheim, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Dainippon Sumitomo, Janssen, Merck Biopharma, MSD, ONO Pharmaceutical Co., Ltd., and Taiho Pharmaceutical; consulting fees for advisory role from Bristol Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, and MSD; honoraria for lectures from Bayer, Chugai, Merck Biopharma, ONO Pharmaceutical Co., Ltd., Taiho Pharmaceutical, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly and company, MSD, Takeda, Asahi Kasei, and Yakult, outside the submitted work. Akihito Tsuji reports research grant to his institution from Taiho Pharmaceutical Co., Ltd., and Chugai Pharmaceutical Co., Ltd., and personal fees from Merck Biopharma Japan, Bristol Myers Squibb, Daiichi Sankyo, Toray Medical Co., Ltd., MSD Corporation, Eli Lilly Japan, Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Hisateru Yasui reports research funding to his institution from MSD, Daiichi Sankyo, ONO Pharmaceutical Co., Ltd., Amgen, and Astellas and honoraria for lectures from Daiichi Sankyo, ONO Pharmaceutical Co., Ltd., Taiho Pharmaceutical, Chugai Pharmaceutical, Bristol Myers Squibb Japan, Terumo, Eli Lilly Japan, Merck Biopharma, Yakult Honsha, Bayer Yakuhin, and Takeda Pharmaceutical, outside the submitted work. Kei Muro reports research funding to his institution from Chugai Pharmaceutical, Amgen, Sanofi, Astellas, Eisai, Novartis, and Pfizer Inc.; consultation fees paid to him from Amgen, ONO Pharmaceutical Co., Ltd., and AstraZeneca; and honoraria for lectures from ONO Pharmaceutical Co., Ltd., Daiichi Sankyo, Taiho Pharmaceutical, Takeda, Bristol Myers Squibb, and Eli Lilly and company, outside the submitted work. Taroh Satoh reports research grant to his institution from Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharmaceutical, MSD, Daiichi Sankyo, BeiGene, Taiho Pharmaceutical, and Janssen and personal fees from ONO Pharmaceutical Co., Ltd., Bristol Myers Squibb, Eli Lilly and company, Daiichi Sankyo, and Taiho Pharmaceutical, outside the submitted work. Takashi Ogata reports receiving a lecture fee from MSD, outside the submitted work. Masahiro Goto reports receiving grants from MSD K.K., during the conduct of the present study and personal fees and non-financial support from ONO Pharmaceutical Co., Ltd., and Taiho Pharmaceutical Co., Ltd., outside the submitted work. Hideo Baba reports receiving research grant from MSD K.K., during the conduct of the present study, grants to his institution from ONO Pharmaceutical Co., Ltd., AstraZeneca K.K., bitBiome, Inc., Yakult Honsha Co., Ltd., Chugai Pharmaceutical Co., Ltd., Shin Nippon Biomedical Laboratories, Ltd., Asahi Kasei Pharma Corporation, Eli Lilly Japan K.K., Johnson & Johnson K.K., Eisai Co., Ltd., and Taiho Pharmaceutical Co., Ltd.; and personal fees from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Daiichi Sankyo Co. Ltd., and Miyarisan Pharmaceutical Co. Ltd., outside the submitted work. Tomohiro Nishina reports grant to his institution from Daiichi Sankyo, ONO Pharmaceutical Co., Ltd., Bristol Myers Squibb, Eily Lilly and Company, Merck Serono, Chugai Pharmaceutical Co., Ltd., and Taiho Pharmaceutical Co., Ltd.; and personal fees from Daiichi Sankyo, ONO Pharmaceutical Co., Ltd., Bristol Myers Squibb, Eily Lilly and Company, Takeda Pharmaceutical Co., Ltd., Merck Serono, Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Yakult Honsha, outside the submitted work. ShiRong Han and Naoyoshi Yatsuzuka are employees of MSD K.K., Tokyo, Japan, and own stock in Merck & Co., Inc., Rahway, NJ, USA. Keiichi Iwakami is an employee of MSD K.K., Tokyo, Japan. Toshihiko Doi reports grants or contracts to his institution from PRA Health Sciences, MSD, Daiichi Sankyo, Amgen, Taiho Pharmaceutical Co., Ltd,, GSK, ONO Pharmaceutical Co., Ltd,, Janssen Pharmaceuticals, Boehringer Ingelheim, Pfizer Inc., Bristol Myers Squibb, AbbVie, Eisai, RIN Institute, Chugai Pharmaceutical Co., Ltd., and Shionogi; consulting fees paid to him from Sumitomo Pharma, Oncolys BioPharma, Takeda Pharmaceutical, Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim, Nano Carrier, Rakuten Medical, Otsuka Pharmaceutical, Kaken Pharmaceutical Co., Ltd., Kyowa Kirin, Shionogi, PRA Health Science, A2 Health Care, and Noil-Immune Biotech Inc; personal payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Daiichi Sankyo; and participation on a data safety monitoring board or advisory board of Gilead Sciences, Pfizer Inc., Amgen, and Zymeworks. Ryu Ishihara reports no disclosures.
Figures



References
-
- Merck Sharp & Dohme Corp. Merck’s Keytruda® (Pembrolizumab) Approved in Japan in combination with chemotherapy for firstline treatment of patients with radically unresectable, Advanced or recurrent esophageal carcinoma. In Kenilworth, Nj: Merck Sharp & Dohme Corp.; 2021.
-
- Merck Sharp & Dohme B.V. Keytruda 25 Mg/Ml concentrate for solution for infusion (Spc), 2022. pp. 130.
-
- Metges J-P, Kato K, Sun J-M, et al. First-line pembrolizumab plus chemotherapy versus chemotherapy in advanced esophageal cancer: longer-term efficacy, safety, and quality-of-life results from the phase 3 keynote-590 study. J Clin Oncol. 2022;40(4_Suppl):241. doi: 10.1200/JCO.2022.40.4_suppl.241. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
