Incidence and Presenting Characteristics of Angiosarcoma in the US, 2001-2020
- PMID: 38607625
- PMCID: PMC11015348
- DOI: 10.1001/jamanetworkopen.2024.6235
Incidence and Presenting Characteristics of Angiosarcoma in the US, 2001-2020
Abstract
Importance: Angiosarcoma is an aggressive vascular malignant neoplasm presenting either as a primary or secondary cancer, often arising after radiotherapy or in the context of preexisting lymphedema. Comprehensive data describing its incidence and presentation patterns are needed.
Objective: To describe the incidence, presenting characteristics, and change over time of angiosarcoma in the US.
Design, setting, and participants: This retrospective cross-sectional study used data from the US Cancer Statistics (USCS) National Program of Cancer Registries-Surveillance, Epidemiology, and End Results Combined Database, which captures more than 99% of newly diagnosed cancers in the US. The study included all 19 289 patients in the US with a new diagnosis of angiosarcoma between 2001 and 2020 captured in the USCS database. Statistical analysis was performed from June to September 2023.
Main outcomes and measures: Incidence of angiosarcoma, demographics of patients with angiosarcoma, and extent of disease at presentation.
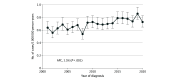
Results: The study included 19 289 patients (median age, 71 years [IQR, 59-80 years]; 10 506 women [54.5%]) with a new diagnosis of angiosarcoma. The US incidence of angiosarcoma doubled between 2001 (657 cases) and 2019 (1312 cases), reflecting both an increase in the adjusted incidence rate of 1.6% per year (P = .001), to 3.3 cases per 1 000 000 person-years (95% CI, 3.1-3.5 cases per 1 000 000 person-years), and an increase in the population at risk. In 2020, the reported incidence rate (3.0 cases per 1 000 000 person-years) and cases of angiosarcoma (n = 1159) were modestly lower than in 2019. Overall, 72.3% of cases of angiosarcoma (n = 13 955) were cutaneous, subcutaneous, or breast angiosarcomas; 24.4% were visceral (n = 4701); and 3.3% were located in unknown or rare primary sites (n = 633). Secondary breast and chest wall angiosarcomas among women represented the largest contribution to increasing incidence. Among breast angiosarcomas, 99.2% (2684 of 2705) were in women and 71.9% (1944 of 2705) were secondary. A total of 80.4% of chest wall or thorax cases among women (1861 of 2316) were secondary vs 26.5% among men (112 of 422), and 63.9% of upper extremity cases among women (205 of 321) were secondary vs 26.8% (56 of 209) among men (P = .001). Rates of secondary angiosarcoma in the abdomen and lower extremities were similar between men and women. The incidence rate of visceral angiosarcoma was also found to be increasing (1.5% per year; P = .001).
Conclusions and relevance: This cross-sectional study describes angiosarcoma presentation patterns and incidence rates in the US over a 20-year period and shows that the number of cases in men and women increased, with the greatest increase among women with secondary angiosarcoma of the chest, breast, and upper extremity. These data increase awareness of a rare but highly morbid disease and highlight the need for improved early detection of angiosarcoma among patients at high risk, such as women with a history of breast cancer.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

