C. elegans Presenilin Mediates Inter-Organelle Contacts and Communication that Is Required for Lysosome Activity
- PMID: 38607730
- PMCID: PMC12339110
- DOI: 10.14336/AD.2024.0228
C. elegans Presenilin Mediates Inter-Organelle Contacts and Communication that Is Required for Lysosome Activity
Abstract
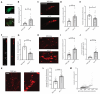
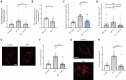
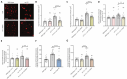
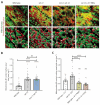
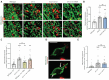
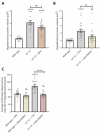
Compromised lysosome function is implicated in the pathology of many neurodegenerative diseases, including Alzheimer's disease (AD). Familial Alzheimer's disease (fAD) is caused primarily by mutations in the presenilin encoding genes, but the underlying mechanism remains obscure. Loss of the conserved C. elegans presenilin orthologue SEL-12 results in increased mitochondrial calcium, which promotes neurodegeneration. Here, we find that sel-12 mutant lysosomes, independent of SEL-12 proteolytic activity, are significantly enlarged and more alkaline due to increased ER-to-mitochondrial calcium signaling and concomitant mitochondrial oxidative stress. These defects and their dependence on mitochondrial calcium are recapitulated in human fAD fibroblasts, demonstrating a conserved role for mitochondrial calcium in presenilin-mediated lysosome dysfunction. sel-12 mutants also have increased contact surface area between the ER, mitochondria, and lysosomes, suggesting sel-12 has an additional role in modulating organelle contact and communication. Overall, we demonstrate that SEL-12 maintains lysosome acidity and lysosome health by controlling ER-to-mitochondrial calcium signaling.
Figures

Similar articles
-
Identification of presenilin mutations that have sufficient gamma-secretase proteolytic activity to mediate Notch signaling but disrupt organelle and neuronal health.Neurobiol Dis. 2025 Aug;212:106961. doi: 10.1016/j.nbd.2025.106961. Epub 2025 May 20. Neurobiol Dis. 2025. PMID: 40404063 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A C. elegans model of familial Alzheimer's disease shows age-dependent synaptic degeneration independent of amyloid β-peptide.bioRxiv [Preprint]. 2025 Jul 20:2025.07.16.665161. doi: 10.1101/2025.07.16.665161. bioRxiv. 2025. PMID: 40791515 Free PMC article. Preprint.
-
Rescue of protein dyshomeostasis in hippocampal astrocytes from an Alzheimer's disease mouse model by stabilizing ER-mitochondrial interactions at a 20 nm distance.Alzheimers Res Ther. 2025 Jul 4;17(1):148. doi: 10.1186/s13195-025-01793-9. Alzheimers Res Ther. 2025. PMID: 40615914 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Identification of presenilin mutations that have sufficient gamma-secretase proteolytic activity to mediate Notch signaling but disrupt organelle and neuronal health.Neurobiol Dis. 2025 Aug;212:106961. doi: 10.1016/j.nbd.2025.106961. Epub 2025 May 20. Neurobiol Dis. 2025. PMID: 40404063 Free PMC article.
-
Mitochondrial Dysfunction in Aging and Age-related Disorders.Aging Dis. 2025 Jul 31;16(5):2495-2497. doi: 10.14336/AD.2025.10731. Aging Dis. 2025. PMID: 40789568 Free PMC article.
References
-
- Kaushik S, Cuervo AM (2015). Proteostasis and aging. Nat Med, 21: 1406-15. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
