Management of Select Adverse Events Following Delandistrogene Moxeparvovec Gene Therapy for Patients With Duchenne Muscular Dystrophy
- PMID: 38607761
- PMCID: PMC11091590
- DOI: 10.3233/JND-230185
Management of Select Adverse Events Following Delandistrogene Moxeparvovec Gene Therapy for Patients With Duchenne Muscular Dystrophy
Abstract
Background: Duchenne muscular dystrophy (DMD) is a rare, degenerative, recessive X-linked neuromuscular disease. Mutations in the gene encoding dystrophin lead to the absence of functional dystrophin protein. Individuals living with DMD exhibit progressive muscle weakness resulting in loss of ambulation and limb function, respiratory insufficiency, and cardiomyopathy, with multiorgan involvement. Adeno-associated virus vector-mediated gene therapy designed to enable production of functional dystrophin protein is a new therapeutic strategy. Delandistrogene moxeparvovec (Sarepta Therapeutics, Cambridge, MA) is indicated for treatment of ambulatory pediatric patients aged 4 through 5 years with DMD who have an indicated mutation in the DMD gene.
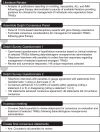
Objective: Evidence-based considerations for management of potential adverse events following gene therapy treatment for DMD are lacking in clinical literature. Our goal was to provide interdisciplinary consensus considerations for selected treatment-related adverse events (TRAEs) (vomiting, acute liver injury, myocarditis, and immune-mediated myositis) that may arise following gene therapy dosing with delandistrogene moxeparvovec.
Methods: An interdisciplinary panel of 12 specialists utilized a modified Delphi process to develop consensus considerations for the evaluation and management of TRAEs reported in delandistrogene moxeparvovec clinical studies. Panelists completed 2 Questionnaires prior to gathering for an in-person discussion. Consensus was defined as a majority (≥58% ; 7/12) of panelists either agreeing or disagreeing.
Results: Panelists agreed that the choice of baseline assessments should be informed by individual clinical indications, the treating provider's judgment, and prescribing information. Corticosteroid dosing for treatment of TRAEs should be optimized by considering individual risk versus benefit for each indication. In all cases involving patients with a confirmed TRAE, consultations with appropriate specialists were suggested.
Conclusions: The Delphi Panel established consensus considerations for the evaluation and management of potential TRAEs for patients receiving delandistrogene moxeparvovec, including vomiting, acute liver injury, myocarditis, and immune-mediated myositis.
Keywords: Duchenne muscular dystrophy; dystrophin; gene therapy; liver injury; myocarditis; myositis; vomiting.
Conflict of interest statement
CMZ: consultancy/advisory role and speaker fees with Sarepta and Optum Therapeutics; research funding from Biogen, Novartis. NLG: consultancy/advisory role with Novartis and Sarepta. AAA: consultancy/advisory role with Mirum Pharma, Albireo/Ipsen, and Sarepta Therapeutics. KDM: consultancy/advisory role with Sarepta Therapeutics, ML Bio; research funding from AMO Pharma, Capricor Therapeutics, Edgewise Therapeutics, FibroGen, Avidity, Italfarmaco, Reata, LEXEO, Biogen, Biohaven, Scholar Rock, PTC Therapeutics, Pfizer, and Sarepta Therapeutics. KNH: consultancy/advisory role with Bayer AG, Bristol-Myers Squibb, Capricor Therapeutics, Catabasis Pharmaceuticals, Daiichi Sankyo, PTC Therapeutics, Revidia Therapeutics, Sarepta Therapeutics, Inc., Stealth Biotherapeutics, Vertex Pharmaceuticals, and Wave Life Science; research funding from Sarepta Therapeutics; speakers’ bureau for NS Pharma and PTC Therapeutics; other relationship(s) with Blade Therapeutics (DSMB) and FibroGen (DSMB). RGC: equity interest in and consultancy/advisory role with LEXEO Therapeutics and XyloCor Therapeutics. AV: consultancy/advisory role with AMO Pharma, AveXis, Biogen, Edgewise Therapeutics, FibroGen, Novartis, Pfizer, PTC Therapeutics, Sarepta Therapeutics, Inc., UCB Pharma and Scholar Rock; research funding from AMO Pharma, Capricor Therapeutics, Edgewise Therapeutics, FibroGen, Muscular Dystrophy Association, Novartis, Parent Project Muscular Dystrophy, Pfizer, RegenxBio, and Sarepta Therapeutics, Inc.; other relationship(s) with MedLink Neurology for editorial services. KEG and EKS: employment with Sarepta Therapeutics, Inc. RJB: consultancy/advisory role with AavantiBio, Biogen, Reata, Sarepta Therapeutics, Inc., and Scholar Rock. AMC: consultancy/advisory role with Biohaven, Edgewise, Sarepta Therapeutics, Inc., and Scholar Rock; research funding from Biohaven, Edgewise, FibroGen, MDA, Sarepta Therapeutics, Inc., and Scholar Rock. CMP: consultancy/advisory role with AveXis/Novartis Gene Therapies, Biogen, Genentech/Roche, Sarepta Therapeutics, Inc., and Scholar Rock; research funding from AveXis/Novartis Gene Therapies, Astellas, Biogen, CSL Behring, FibroGen, PTC, Pfizer, Sarepta, and Scholar Rock; speakers’ bureau for Biogen. PBW: consultancy/advisory role with Alnylam, Astellas, Bayer, Biogen, Fore Therapeutics, RegenxBio, Roche, Sarepta, Intellia Therapeutics, Pfizer, and Verve Therapeutics. JRM: financial support from Sarepta Therapeutics, Inc., for the travel to meetings to present products sponsored by Sarepta; research funding from Sarepta Therapeutics, Inc.; patents, royalties, or other intellectual property as co-inventor of AAVrh74.MHCK; and is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer review.
Figures

References
-
- Barohn RJ, Levine EJ, Olson JO, Mendell JR. Gastric hypomotility in Duchenne’s muscular dystrophy. New Engl J Med. 1988;319(1):15–8. - PubMed
-
- Kononets O, Karaiev T, Tkachenko O, Lichman L. Renal, hepatic and immune function indices in patients with Duchenne muscular dystrophy. Georgian Med News. 2020;(309);64–71. - PubMed
-
- Hellebrekers DMJ, van Abeelen SAM, Catsman CE, van Kuijk SMJ, Laridon AM, Klinkenberg S, et al. Cognitive and behavioral functioning in two neurogenetic disorders; how different are these aspects in Duchenne muscular dystrophy and Neurofibromatosis type 1? PLoS One. 2022;17(10):e0275803. - PMC - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol (2010) 9(1), 77–93. - PubMed
-
- Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: One gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2(12):731–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

