Extracellular vesicles released by keratinocytes regulate melanosome maturation, melanocyte dendricity, and pigment transfer
- PMID: 38607931
- PMCID: PMC11032449
- DOI: 10.1073/pnas.2321323121
Extracellular vesicles released by keratinocytes regulate melanosome maturation, melanocyte dendricity, and pigment transfer
Abstract
Extracellular vesicles (EVs) facilitate the transfer of proteins, lipids, and genetic material between cells and are recognized as an additional mechanism for sustaining intercellular communication. In the epidermis, the communication between melanocytes and keratinocytes is tightly regulated to warrant skin pigmentation. Melanocytes synthesize the melanin pigment in melanosomes that are transported along the dendrites prior to the transfer of melanin pigment to keratinocytes. EVs secreted by keratinocytes modulate pigmentation in melanocytes [(A. Lo Cicero et al., Nat. Commun. 6, 7506 (2015)]. However, whether EVs secreted by keratinocytes contribute to additional processes essential for melanocyte functions remains elusive. Here, we show that keratinocyte EVs enhance the ability of melanocytes to generate dendrites and mature melanosomes and promote their efficient transfer. Further, keratinocyte EVs carrying Rac1 induce important morphological changes, promote dendrite outgrowth, and potentiate melanin transfer to keratinocytes. Hence, in addition to modulating pigmentation, keratinocytes exploit EVs to control melanocyte plasticity and transfer capacity. These data demonstrate that keratinocyte-derived EVs, by regulating melanocyte functions, are major contributors to cutaneous pigmentation and expand our understanding of the mechanism underlying skin pigmentation via a paracrine EV-mediated communication.
Keywords: dendrites; melanocytes; pigment transfer; skin pigmentation; small extracellular vesicles.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
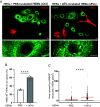
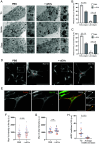
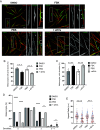
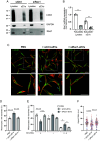
References
-
- Fitzpatrick T. B., Breathnach A. S., The epidermal melanin unit system. Dermatol. Wochenschr. 147, 481–489 (1963). - PubMed
-
- Yamaguchi Y., Brenner M., Hearing V. J., The regulation of skin pigmentation. J. Biol. Chem. 282, 27557–27561 (2007). - PubMed
-
- Tsakraklides V., et al. , Subcellular localization of GFP-myosin-V in live mouse melanocytes. J. Cell Sci. 112, 2853–2865 (1999). - PubMed
MeSH terms
Substances
Grants and funding
- EQU201903007827/Fondation pour la Recherche Médicale (FRM)
- 847718/EC | H2020 | PRIORITY 'Excellent science' | H2020 Marie Skłodowska-Curie Actions (MSCA)
- ANR10-INBS-04/Association Nationale de la Recherche
- SPF201809006932/Fondation pour la Recherche Médicale (FRM)
- 847718/Institut Curie EuReCa PhD Programme
LinkOut - more resources
Full Text Sources
Research Materials

