Neddylation orchestrates the complex transcriptional and posttranscriptional program that drives Schwann cell myelination
- PMID: 38608019
- PMCID: PMC11014456
- DOI: 10.1126/sciadv.adm7600
Neddylation orchestrates the complex transcriptional and posttranscriptional program that drives Schwann cell myelination
Abstract
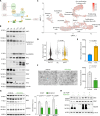
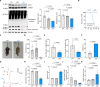
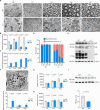
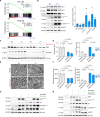
Myelination is essential for neuronal function and health. In peripheral nerves, >100 causative mutations have been identified that cause Charcot-Marie-Tooth disease, a disorder that can affect myelin sheaths. Among these, a number of mutations are related to essential targets of the posttranslational modification neddylation, although how these lead to myelin defects is unclear. Here, we demonstrate that inhibiting neddylation leads to a notable absence of peripheral myelin and axonal loss both in developing and regenerating mouse nerves. Our data indicate that neddylation exerts a global influence on the complex transcriptional and posttranscriptional program by simultaneously regulating the expression and function of multiple essential myelination signals, including the master transcription factor EGR2 and the negative regulators c-Jun and Sox2, and inducing global secondary changes in downstream pathways, including the mTOR and YAP/TAZ signaling pathways. This places neddylation as a critical regulator of myelination and delineates the potential pathogenic mechanisms involved in CMT mutations related to neddylation.
Figures




References
-
- Fledrich R., Kungl T., Nave K. A., Stassart R. M., Axo-glial interdependence in peripheral nerve development. Development 146, (2019). - PubMed
-
- Pipis M., Rossor A. M., Laura M., Reilly M. M., Next-generation sequencing in Charcot-Marie-Tooth disease: Opportunities and challenges. Nat. Rev. Neurol. 15, 644–656 (2019). - PubMed
-
- Suter U., Scherer S. S., Disease mechanisms in inherited neuropathies. Nat. Rev. Neurosci. 4, 714–726 (2003). - PubMed
-
- Petroski M. D., Deshaies R. J., Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6, 9–20 (2005). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

