Interlaboratory comparison of a multiplex immunoassay that measures human serum IgG antibodies against six-group B streptococcus polysaccharides
- PMID: 38608170
- PMCID: PMC11018077
- DOI: 10.1080/21645515.2024.2330138
Interlaboratory comparison of a multiplex immunoassay that measures human serum IgG antibodies against six-group B streptococcus polysaccharides
Abstract
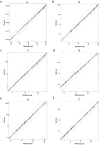
Measurement of IgG antibodies against group B streptococcus (GBS) capsular polysaccharide (CPS) by use of a standardized and internationally accepted multiplex immunoassay is important for the evaluation of candidate maternal GBS vaccines in order to compare results across studies. A standardized assay is also required if serocorrelates of protection against invasive GBS disease are to be established in infant sera for the six predominant GBS serotypes since it would permit the comparison of results across the six serotypes. We undertook an interlaboratory study across five laboratories that used standardized assay reagents and protocols with a panel of 44 human sera to measure IgG antibodies against GBS CPS serotypes Ia, Ib, II, III, IV, and V. The within-laboratory intermediate precision, which included factors like the lot of coated beads, laboratory analyst, and day, was generally below 20% relative standard deviation (RSD) for all six serotypes, across all five laboratories. The cross-laboratory reproducibility was < 25% RSD for all six serotypes, which demonstrated the consistency of results across the different laboratories. Additionally, anti-CPS IgG concentrations for the 44-member human serum panel were established. The results of this study showed assay robustness and that the resultant anti-CPS IgG concentrations were reproducible across laboratories for the six GBS CPS serotypes when the standardized assay was used.
Keywords: Group B streptococcus; correlate of protection; maternal; neonatal; vaccines.
Conflict of interest statement
Authors who are employees of Pfizer may, as a consequence, be shareholders of Pfizer Inc.
Figures
References
-
- Gonçalves BP, Procter SR, Paul P, Chandna J, Lewin A, Seedat F, Koukounari A, Dangor Z, Leahy S, Santhanam S, et al. Group B streptococcus infection during pregnancy and infancy: estimates of regional and global burden. Lancet Glob Health. 2022. Jun;10(6):e807–7. doi:10.1016/S2214-109X(22)00093-6. - DOI - PMC - PubMed
-
- Absalon J, Simon R, Radley D, Giardina PC, Koury K, Jansen KU, Anderson AS.. Advances towards licensure of a maternal vaccine for the prevention of invasive group B streptococcus disease in infants: a discussion of different approaches. Hum Vaccin Immunother. 2022 Dec 31;18(1):2037350. doi:10.1080/21645515.2022.2037350. - DOI - PMC - PubMed
-
- Siber GR, Chang I, Baker S, Fernsten P, O’Brien KL, Santosham M, Klugman KP, Madhi SA, Paradiso P, Kohberger R, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007 May 10;25(19):3816–26. doi:10.1016/j.vaccine.2007.01.119. - DOI - PubMed
-
- Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN, et al. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016 Dec 3;388(10061):2775–2782. doi:10.1016/S0140-6736(16)31921-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
