Machine Learning-Based Survival Prediction Models for Progression-Free and Overall Survival in Advanced-Stage Hodgkin Lymphoma
- PMID: 38608215
- PMCID: PMC11161240
- DOI: 10.1200/CCI.23.00255
Machine Learning-Based Survival Prediction Models for Progression-Free and Overall Survival in Advanced-Stage Hodgkin Lymphoma
Abstract
Purpose: Patients diagnosed with advanced-stage Hodgkin lymphoma (aHL) have historically been risk-stratified using the International Prognostic Score (IPS). This study investigated if a machine learning (ML) approach could outperform existing models when it comes to predicting overall survival (OS) and progression-free survival (PFS).
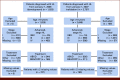
Patients and methods: This study used patient data from the Danish National Lymphoma Register for model development (development cohort). The ML model was developed using stacking, which combines several predictive survival models (Cox proportional hazard, flexible parametric model, IPS, principal component, penalized regression) into a single model, and was compared with two versions of IPS (IPS-3 and IPS-7) and the newly developed aHL international prognostic index (A-HIPI). Internal model validation was performed using nested cross-validation, and external validation was performed using patient data from the Swedish Lymphoma Register and Cancer Registry of Norway (validation cohort).
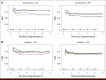
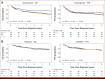
Results: In total, 707 and 760 patients with aHL were included in the development and validation cohorts, respectively. Examining model performance for OS in the development cohort, the concordance index (C-index) for the ML model, IPS-7, IPS-3, and A-HIPI was found to be 0.789, 0.608, 0.650, and 0.768, respectively. The corresponding estimates in the validation cohort were 0.749, 0.700, 0.663, and 0.741. For PFS, the ML model achieved the highest C-index in both cohorts (0.665 in the development cohort and 0.691 in the validation cohort). The time-varying AUCs for both the ML model and the A-HIPI were consistently higher in both cohorts compared with the IPS models within the first 5 years after diagnosis.
Conclusion: The new prognostic model for aHL on the basis of ML techniques demonstrated a substantial improvement compared with the IPS models, but yielded a limited improvement in predictive performance compared with the A-HIPI.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
Similar articles
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
External validation of a machine learning prediction model for massive blood loss during surgery for spinal metastases: a multi-institutional study using 880 patients.Spine J. 2025 Jul;25(7):1386-1399. doi: 10.1016/j.spinee.2025.03.018. Epub 2025 Mar 27. Spine J. 2025. PMID: 40157430
-
Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma.Cochrane Database Syst Rev. 2017 May 25;5(5):CD007941. doi: 10.1002/14651858.CD007941.pub3. Cochrane Database Syst Rev. 2017. PMID: 28541603 Free PMC article.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
Cited by
-
Newly Diagnosed Classical Hodgkin Lymphoma: Optimizing Outcomes in a New Therapeutic Era.Hematol Oncol. 2025 Jun;43 Suppl 2(Suppl 2):e70066. doi: 10.1002/hon.70066. Hematol Oncol. 2025. PMID: 40517538 Free PMC article. Review.
-
Development of time to event prediction models using federated learning.BMC Med Res Methodol. 2025 May 26;25(1):143. doi: 10.1186/s12874-025-02598-y. BMC Med Res Methodol. 2025. PMID: 40419965 Free PMC article.
-
The A-HIPI prediction model in advanced-stage Hodgkin lymphoma: identification of risk groups and creation of an online tool.Blood Adv. 2025 Mar 25;9(6):1366-1369. doi: 10.1182/bloodadvances.2024014689. Blood Adv. 2025. PMID: 39778124 Free PMC article. No abstract available.
-
Hematological Malignancies in Older Patients: Focus on the Potential Role of a Geriatric Assessment Management.Diagnostics (Basel). 2024 Jun 29;14(13):1390. doi: 10.3390/diagnostics14131390. Diagnostics (Basel). 2024. PMID: 39001280 Free PMC article. Review.
-
External validation and calibration of the HoLISTIC Consortium's advanced-stage Hodgkin lymphoma international prognostic index (A-HIPI) in the Brazilian Hodgkin lymphoma registry.Br J Haematol. 2025 Jan;206(1):144-151. doi: 10.1111/bjh.19824. Epub 2024 Oct 17. Br J Haematol. 2025. PMID: 39419492
References
-
- Eichenauer DA, Aleman BMP, André M, et al. : Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv19-iv29, 2018 - PubMed
-
- Swerdlow SH: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues Revised 4. Lyon, International Agency for Research on Cancer, 2017
-
- Biccler JL, Glimelius I, Eloranta S, et al. : Relapse risk and loss of lifetime after modern combined modality treatment of young patients with Hodgkin lymphoma: A Nordic Lymphoma Epidemiology Group Study. J Clin Oncol 37:703-713, 2019 - PubMed
-
- Hasenclever D, Diehl V, Armitage JO, et al. : A prognostic score for advanced Hodgkin's disease: International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med 339:1506-1514, 1998 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

