The 2022 review of the 2019 American Society of Hematology guidelines on immune thrombocytopenia
- PMID: 38608258
- PMCID: PMC11319830
- DOI: 10.1182/bloodadvances.2023012541
The 2022 review of the 2019 American Society of Hematology guidelines on immune thrombocytopenia
Abstract
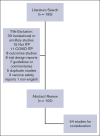
The 2019 American Society of Hematology (ASH) guidelines for immune thrombocytopenia (ITP) included recommendations on the management of adults (recommendations 1-9) and children (recommendations 10-21) with primary ITP . We describe here the results of a review of the 2019 guidelines by a working group of experts requested by ASH to inform decision-making about the need for and timing of a guideline revision. An updated Medline and Embase search applied the same search terms as in the 2019 ASH guidelines, limited to systematic reviews and clinical trials, from May 2017 to July 2022. There were 193 studies identified, 102 underwent abstract reviews, and 54 full reviews. Each study was assessed based on relevance to the previous recommendation with regard to the population, prioritized outcomes, new outcomes, and study design. Reviewers assessed if the data would change the strength or the directionality of the existing recommendation or merit development of a new recommendation. Based on this review, the ASH Committee on Quality endorsed a focused update on second-line management for adults with ITP. In addition, there will be continued annual monitoring and reviewing of the 2019 ASH guidelines on ITP in full to evaluate when there is sufficient new evidence to warrant additional revisions.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.E.N. has received research funding from Novartis; has served as a consultant to Novartis, Argenx, and Sanofi; received educational funding from Sobi; received royalties from UpToDate; has served as a medical adviser to UK ITP Support Association; and has voluntarily served ITP Australia. D.M.A. has received research funding from Novartis and Rigel; has served as a consultant to Novartis, Rigel, Amgen, Medison, Daiichi Sankyo, Sobi, Chugai, Principia, Takeda, Cellphire, Alpine, Argenx, and Sanofi; has received royalties from UpToDate; and has served as an honorary medical adviser to Platelet Disorders Support Association. R.F.G. has received research funding from Novartis, Sobi, and Agios; has served on the advisory board of Agios and Sanofi; and has served as an honorary medical adviser to Platelet Disorders Support Association. T.K. has received research funding from Amgen and Novartis; and has served on the advisory board of Argenx, Sobi, and UCB. K.R.M. has served on the data safety board of Argenex and has also served on the advisory board of Novartis and Alpine Bioscience. D.R.T. has served on the advisory board of Sanofi.
Comment in
-
Immune thrombocytopenia guidelines get an annual checkup.Blood Adv. 2024 Jul 9;8(13):3576-3577. doi: 10.1182/bloodadvances.2024013317. Blood Adv. 2024. PMID: 38980671 Free PMC article. No abstract available.
References
-
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr., Crowther MA, American Society of Hematology The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous