Parasympathetic neurons derived from human pluripotent stem cells model human diseases and development
- PMID: 38608707
- PMCID: PMC11069445
- DOI: 10.1016/j.stem.2024.03.011
Parasympathetic neurons derived from human pluripotent stem cells model human diseases and development
Abstract
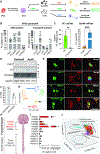
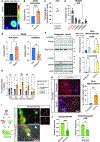
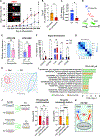
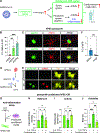
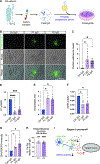
Autonomic parasympathetic neurons (parasymNs) control unconscious body responses, including "rest-and-digest." ParasymN innervation is important for organ development, and parasymN dysfunction is a hallmark of autonomic neuropathy. However, parasymN function and dysfunction in humans are vastly understudied due to the lack of a model system. Human pluripotent stem cell (hPSC)-derived neurons can fill this void as a versatile platform. Here, we developed a differentiation paradigm detailing the derivation of functional human parasymNs from Schwann cell progenitors. We employ these neurons (1) to assess human autonomic nervous system (ANS) development, (2) to model neuropathy in the genetic disorder familial dysautonomia (FD), (3) to show parasymN dysfunction during SARS-CoV-2 infection, (4) to model the autoimmune disease Sjögren's syndrome (SS), and (5) to show that parasymNs innervate white adipocytes (WATs) during development and promote WAT maturation. Our model system could become instrumental for future disease modeling and drug discovery studies, as well as for human developmental studies.
Keywords: COVID-19; Schwann cell progenitors; Sjögren’s syndrome; adipocyte innervation; autonomic nervous system; disease modeling; familial dysautonomia; human pluripotent stem cells; parasympathetic neurons; peripheral nervous system.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A US patent application entitled “Composition and methods for making parasympathetic neurons” was filed under U.S.S.N. 18/503,100.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

