Airway clearance management in people with bronchiectasis: data from the European Bronchiectasis Registry (EMBARC)
- PMID: 38609097
- PMCID: PMC11154755
- DOI: 10.1183/13993003.01689-2023
Airway clearance management in people with bronchiectasis: data from the European Bronchiectasis Registry (EMBARC)
Erratum in
-
"Airway clearance management in people with bronchiectasis: data from the European Bronchiectasis Registry (EMBARC)." A. Spinou, B. Hererro-Cortina, S. Aliberti, et al. Eur Respir J 2024; 63: 2301689.Eur Respir J. 2024 Jul 25;64(1):2351689. doi: 10.1183/13993003.51689-2023. Print 2024 Jul. Eur Respir J. 2024. PMID: 39054041 Free PMC article. No abstract available.
Abstract
Background: International guidelines recommend airway clearance management as one of the important pillars of bronchiectasis treatment. However, the extent to which airway clearance is used for people with bronchiectasis in Europe is unclear. The aim of the study was to identify the use of airway clearance management in patients with bronchiectasis across different countries and factors influencing airway clearance use.
Methods: This was a prospective observational study using data from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) Registry between January 2015 and April 2022. Prespecified options for airway clearance management were recorded, including airway clearance techniques, devices and use of mucoactive drugs.
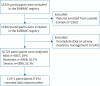
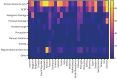
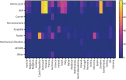
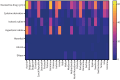
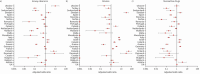
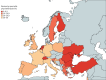
Results: 16 723 people with bronchiectasis from 28 countries were included in the study. The mean age was 67 years (interquartile range 57-74 years, range 18-100 years) and 61% were female. 72% of the participants reported daily sputum expectoration and 52% (95% CI 51-53%) of all participants reported using regular airway clearance management. Active cycle of breathing technique was used by 28% of the participants and airway clearance devices by 16% of participants. The frequency of airway clearance management and techniques used varied significantly between different countries. Participants who used airway clearance management had greater disease severity and worse symptoms, including a higher daily sputum volume, compared to those who did not use it regularly. Mucoactive drugs were also more likely to be used in participants with more severe disease. Access to specialist respiratory physiotherapy was low throughout Europe, but particularly low in Eastern Europe.
Conclusions: Only a half of people with bronchiectasis in Europe use airway clearance management. Use of and access to devices, mucoactive drugs and specialist chest physiotherapy appears to be limited in many European countries.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: B. Hererro-Cortina reports payment or honoraria for lectures, presentations, manuscript writing or educational events from SEPAR (Spanish Respiratory Society), and has a leadership or fiduciary role in SEPAR. S. Aliberti reports grants from Insmed Incorporated, Chiesi, Fisher and Paykel, and GSK; royalties or licences from McGraw Hill; consulting fees from Insmed Incorporated, Insmed Italy, Insmed Ireland Ltd, Zambon Spa, AstraZeneca UK Ltd, AstraZeneca Pharmaceutical LP, CSL Behring GmbH, Grifols, Fondazione Internazionale Menarini, Moderna, Chiesi, MCD Italis Srl, Brahms, Physioassist SAS and GSK SpA; payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK SpA, Thermofisher Scientific, Insmed Italy, Insmed Ireland, Zambon and Fondazione Internazionale Menarini; and participation on a data safety monitoring board or advisory board for Insmed Incorporated, Insmed Italy, AstraZeneca UK Ltd and MSD Italia Srl. P.C. Goeminne reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed, GSK and Chiesi; support for attending meetings and/or travel from Chiesi; and participation on a data safety monitoring board or advisory board for Boehringer, GSK and Pfizer. E. Polverino reports grants from Grifols; consulting fees from Insmed, Bayer, Chiesi and Zambon; payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer, Chiesi, Grifols, GSK, Insmed, Menarini and Zambon; and support for attending meetings and/or travel from Insmed, Pfizer and Moderna. K. Dimakou reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Novartis, Boehringer Ingelheim, GSK, Norma Hellas, Chiesi, AstraZeneca and Zambon; support for attending meetings and/or travel from Novartis, Boehringer Ingelheim, GSK, Norma Hellas, Chiesi, AstraZeneca and Menarini, and participation on a data safety monitoring board or advisory board for Novartis, GSK and Chiesi. C.S. Haworth reports payment or honoraria for lectures, presentations, manuscript writing or educational events from 30 Technology, CSL Behring, Chisi, Insmed, Janssen, LifeArc, Meiji, Mylan, Pneumagen, Shionogi, Vertex and Zambon. M.R. Loebinger reports consulting fees from Armata, 30T, AstraZeneca, Parion, Insmed, Chiesi, Zambon, Electromed, Recode, AN2 and Boehringer Ingelheim; payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed; and is ERS Infection Group Chair. A. De Soyza reports grants from AstraZeneca, Pfizer, GSK and Novartis; consulting fees from AstraZeneca, Insmed, GSK, Boehringer, 30T and Bayer; and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Pfizer, GSK and Novartis. M. Vendrell reports grants from Chiesi; payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed and Publi Creation; support for attending meetings and/or travel from Zambon, Chiesi, Novartis, Behring and Gebro; participation on a data safety monitoring board or advisory board for Insmed; and receipt of equipment, materials, drugs, medical writing, gifts or services from Insmed and Novartis. P.R. Burgel reports grants from GSK and Vertex; consulting fees from AstraZeneca, Chiesi, GSK, Insmed, MSD, Vertex, Viatris and Zambon; and support for attending meetings and/or travel from AstraZeneca and Chiesi. S. Sutharsan reports grants from Vertex, Galapagos, Insmed, Proteostasis and Corbus; consulting fees from Insmed, Vertex Pharmaceuticals and Boehringer Ingelheim; and payment or honoraria for lectures, presentations, manuscript writing or educational events from Vertex Pharmaceuticals, Boehringer Ingelheim and Insmed. S. Škrgat reports honoraria for educational events, invited lectures and presentations supported by Sanofi, AstraZeneca, Medis, Berlin-Chemie and Chiesi; and participation on a data safety monitoring board or advisory board for AstraZeneca. D. Stolz reports payment or honoraria for lectures, presentations, manuscript writing or educational events from CSL Behring, Berlin-Chemie, Menarini, Novartis, GSK, AstraZeneca, Vifor, Merck, Chiesi and Sanofi; and participation on a data safety monitoring board or advisory board for GSK and CSL Behring. P. Kauppi reports support for attending meetings and/or travel from Nordic Respiratory Academy; participation on a data safety monitoring board or advisory board for Swedish Orphan Biovitrium; a leadership or fiduciary role for the Finnish Respiratory Society and Finnish Tuberculosis Foundation Grant Committee; and receipt of equipment, materials, drugs, medical writing, gifts or services from Theravance. A. Bossios reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Chiesi; and is Secretary of Assembly 5 (Airway diseases, asthma, COPD and chronic cough) of the ERS, Vice-chair of the Nordic Severe Asthma Network (NSAN)-NORDSTAR and a member of the Steering Committee of the ERS CRC for severe asthma (SHARP). I. Clifton reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca and GSK; and participation on a data safety monitoring board or advisory board for AstraZeneca, GSK and Infex Therapeutics. M.L. Crichton reports consulting fees from Boxer Capital LLC. P. Walker is Chair of the British Thoracic Society. D. Obradovic is President of the Serbian Society of Intensive Care Medicine. A. Amorim reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Zambon Group, and support for attending meetings and or/travel from Zambon Group, Boehringer Ingelheim and Novartis Farma. F. Blasi reports grants from AstraZeneca, Chiesi and Insmed; consulting fees from Menarini; and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Chiesi, GSK, Guidotti, Grifols, Insmed, Menarini, Novartis, OM Pharma, Pfizer, Sanofi, Viatris, Vertex and Zambon. M. Jankovic Makek reports consulting fees from Insmed, Biomerieux and MSD; payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed, MSD, Pfizer and Teva; and support for attending meetings and/or travel from MSD, Pfizer and Berlin-Chemie. M. Shteinberg reports consulting fees from GSK, Boehringer Ingelheim, Kamada and Zambon; payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed, Boehringer Ingelheim, GSK, AstraZeneca, Teva, Novartis, Kamada and Sanofi; support for attending meetings and/or travel from Novartis, Actelion, Boehringer Ingelheim, GSK and Rafa; participation on a data safety monitoring board or advisory board for Bonus Therapeutics, Israel; leadership or fiduciary roles for EMBARC Management, Israel Pulmonology Society Board and the Israel Society for TB and Mycobacterial Diseases; receipt of equipment, materials, drugs, medical writing, gifts or services from Trudell Medical Int.; and is and Associate Editor of the American Journal of Respiratory and Critical Care Medicine. J.D. Chalmers reports grants from AstraZeneca, Boehringer Ingelheim, Genentech, Gilead Sciences, GSK, Grifols, Insmed, LifeArc and Novartis; and consulting fees from AstraZeneca, Chiesi, GSK, Insmed, Grifols, Novartis, Boehringer Ingelheim, Pfizer, Janssen, Antabio and Zambon. F.C. Ringshausen reports grants from German Center for Lung Research (DZL), German Center for Infection Research (DZIF), IMI (EU/EFPIA) and iABC Consortium (including Alaxia, Basilea, Novartis and Polyphor), Mukoviszidose Institute, Novartis, Insmed Germany, Grifols, Bayer and InfectoPharm; consulting fees from Parion, Grifols, Zambon, Insmed and Helmholtz-Zentrum für Infektionsforschung; payment or honoraria for lectures, presentations, manuscript writing or educational events from I!DE Werbeagentur GmbH, Interkongress GmbH, AstraZeneca, Insmed, Grifols and Universitätsklinikum Frankfurt am Main; payment for expert testimony from Social Court Cologne; support for attending meetings and/or travel from German Kartagener Syndrome and Primary Ciliary Dyskinesia Patient Advocacy Group Mukoviszidose e.V.; participation on a data safety monitoring board or advisory board for Insmed, Grifols and Shionogi; leadership or fiduciary roles as Coordinator of the ERN-LUNG Bronchiectasis Core Network, Chair of the German Bronchiectasis Registry PROGNOSIS, Member of the Steering Committee of the European Bronchiectasis Registry EMBARC, Member of the Steering Committee of the European Nontuberculous Mycobacterial Pulmonary Disease Registry EMBARC-NTM, Co-Speaker of the Medical Advisory Board of the German Kartagener Syndrome and PCD Patient Advocacy Group, Speaker of the Respiratory Infections and TB group of the German Respiratory Society, Speaker of the Cystic Fibrosis group of German Respiratory Society (DGP), principal investigator of the German Center for Lung Research, Member of the Protocol Review Committee of the PCD-CTN and Member of Physician Association of the German Cystic Fibrosis Patient Advocacy Group; and other financial or non-financial interests in AstraZeneca, Boehringer Ingelheim, Celtaxsys, Corbus, Insmed, Novartis, Parion, University of Dundee, Vertex and Zambon. The remaining authors have no potential conflicts of interest to disclose.
Figures
Comment in
-
Mind the gap: challenges to overcome in airway clearance.Eur Respir J. 2024 Jun 6;63(6):2400687. doi: 10.1183/13993003.00687-2024. Print 2024 Jun. Eur Respir J. 2024. PMID: 38843939 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
