Zoledronate After Denosumab Discontinuation: Is Repeated Administrations More Effective Than Single Infusion?
- PMID: 38609157
- PMCID: PMC11403318
- DOI: 10.1210/clinem/dgae224
Zoledronate After Denosumab Discontinuation: Is Repeated Administrations More Effective Than Single Infusion?
Erratum in
-
Correction to: "Zoledronate After Denosumab Discontinuation: Is Repeated Administrations More Effective Than Single Infusion?".J Clin Endocrinol Metab. 2024 Sep 16;109(10):e1986. doi: 10.1210/clinem/dgae429. J Clin Endocrinol Metab. 2024. PMID: 38949949 Free PMC article. No abstract available.
Abstract
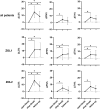
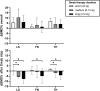
Background: After denosumab (Dmab) discontinuation C-terminal telopeptide (CTX) levels increase, bone mineral density (BMD) decreases and multiple vertebral fractures (FX) may occur with relevant impacts on women's health. A sequential therapy with bisphosphonates is recommended, and the European Calcified Tissue Society (ECTS) proposed repeated zoledronate (ZOL) administrations in patients with persistently high CTX levels, although the efficacy of this schedule is unknown. In this retrospective study, we describe BMD changes and FX rate in 52 patients managed according to the ECTS recommendations.
Methods: We measured CTX levels and administered ZOL after 1 month from Dmab withdrawal (t0). After 6 months (t1), we administered a second ZOL infusion, if CTX levels were ≥280 ng/L. BMD changes and FX rate were assessed on average after 17 months from Dmab withdrawal.
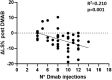
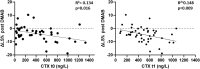
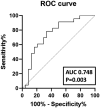
Results: Seventy-five percent of patients repeated ZOL infusion. In this group, spine BMD declined significantly (-5.5 ± 5.6%), while it remained stable in the group with CTX levels <280 ng/L (-0.1 ± 5.5%, P = 0.008). All fractured patients (9.6%) had received >5 Dmab injections and 2 ZOL infusions. The BMD worsening after Dmab withdrawal was associated with CTX t1 [odds ratio (OR) 2.9, interquartile range (IQR) 1.3-6.6, P = .009] and spine BMD gain during Dmab therapy corrected for the number of Dmab injections (OR 3.0, IQR 1.2-7.2, P = .014). A CTX level at t1 > 212 ng/L had 100% sensitivity in predicting the BMD loss.
Conclusion: In patients with uncontrolled CTX levels after Dmab withdrawal, 2 ZOL infusions 6 months apart do not prevent BMD loss and FX.
Keywords: bone turnover markers; denosumab withdrawal; osteoporosis; rebound effect; sequential therapy; zoledronate.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
-
- Bone HG, Wagman RB, Brandi ML, et al. . 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513‐523. - PubMed
-
- Lacey DL, Boyle WJ, Simonet WS, et al. . Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401‐419. - PubMed
-
- Bone HG, Bolognese MA, Yuen CK, et al. . Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972‐980. - PubMed
-
- Zanchetta MB, Boailchuk J, Massari F, Silveira F, Bogado C, Zanchetta JR. Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study. Osteoporos Int. 2018;29(1):41‐47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

