Nr1h4 and Thrb ameliorate ER stress and provide protection in the MPTP mouse model of Parkinson's
- PMID: 38609183
- PMCID: PMC11015051
- DOI: 10.26508/lsa.202302416
Nr1h4 and Thrb ameliorate ER stress and provide protection in the MPTP mouse model of Parkinson's
Abstract
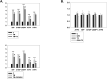
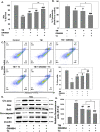
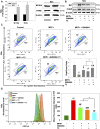
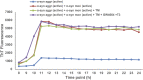
Elevated ER stress has been linked to the pathogenesis of several disease conditions including neurodegeneration. In this study, we have holistically determined the differential expression of all the nuclear receptors (NRs) in the presence of classical ER stress inducers. Activation of Nr1h4 and Thrb by their cognate ligands (GW4064 and T3) ameliorates the tunicamycin (TM)-induced expression of ER stress genes. A combination of both ligands is effective in mitigating cell death induced by TM. Further exploration of their protective effects in the Parkinson's disease (PD) model shows that they reduce MPP+-induced dissipation of mitochondrial membrane potential and ROS generation in an in vitro PD model in neuronal cells. Furthermore, the generation of an experimental murine PD model reveals that simultaneous treatment of GW4064 and T3 protects mice from ER stress, dopaminergic cell death, and functional deficits in the MPTP mouse model of PD. Thus, activation of Nr1h4 and Thrb by their respective ligands plays an indispensable role in ER stress amelioration and mounts protective effects in the MPTP mouse model of PD.
© 2024 Ahuja et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures









Similar articles
-
MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration.Cell Death Differ. 2019 Jan;26(2):213-228. doi: 10.1038/s41418-018-0124-5. Epub 2018 May 21. Cell Death Differ. 2019. PMID: 29786072 Free PMC article.
-
Downregulation of miR-124 in MPTP-treated mouse model of Parkinson's disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/cdk5 pathway proteins.Neuroscience. 2014 Jul 11;272:167-79. doi: 10.1016/j.neuroscience.2014.04.039. Epub 2014 Apr 30. Neuroscience. 2014. PMID: 24792712
-
Impaired CBS-H2S signaling axis contributes to MPTP-induced neurodegeneration in a mouse model of Parkinson's disease.Brain Behav Immun. 2018 Jan;67:77-90. doi: 10.1016/j.bbi.2017.07.159. Epub 2017 Aug 1. Brain Behav Immun. 2018. PMID: 28774789
-
alpha-Synuclein- and MPTP-generated rodent models of Parkinson's disease and the study of extracellular striatal dopamine dynamics: a microdialysis approach.CNS Neurol Disord Drug Targets. 2010 Aug;9(4):482-90. doi: 10.2174/187152710791556177. CNS Neurol Disord Drug Targets. 2010. PMID: 20522009 Review.
-
The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease.Curr Pharm Des. 2011;17(5):489-507. doi: 10.2174/138161211795164095. Curr Pharm Des. 2011. PMID: 21375482 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
