Phase 1 study combining elotuzumab with autologous stem cell transplant and lenalidomide for multiple myeloma
- PMID: 38609316
- PMCID: PMC11029259
- DOI: 10.1136/jitc-2023-008110
Phase 1 study combining elotuzumab with autologous stem cell transplant and lenalidomide for multiple myeloma
Abstract
Background: Autologous stem cell transplantation (ASCT) after induction therapy improves disease-free survival for patients with multiple myeloma (MM). While the goal of ASCT is to render a minimal disease state, it is also associated with eradication of immunosuppressive cells, and we hypothesize that early introduction of immunotherapy post-ASCT may provide a window of opportunity to boost treatment efficacy.
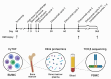
Methods: We conducted a phase 1 clinical trial to investigate the application of autologous lymphocyte infusion and anti-SLAMF7 monoclonal antibody, elotuzumab, after ASCT in patients with newly diagnosed MM previously treated with induction therapy. In addition to CD34+ stem cells, peripheral blood mononuclear cells were harvested prior to transplant and infused on day 3 after stem cell infusion to accelerate immune reconstitution and provide autologous natural killer (NK) cells that are essential to the mechanism of elotuzumab. Elotuzumab was administered starting on day 4 and then every 28 days after until 1 year post-ASCT. Cycles 4-12 were administered with standard-of-care lenalidomide maintenance.
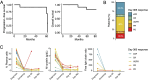
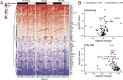
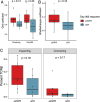
Results: All subjects were evaluated for safety, and 13 of 15 subjects completed the treatment protocol. At 1 year post-ASCT, the disease status of enrolled subjects was as follows: five stringent complete responses, one complete response, six very good partial responses, one partial response, and two progressive diseases. The treatment plan was well tolerated, with most grade 3 and 4 AEs being expected hematologic toxicities associated with ASCT. Correlative analysis of the immune microenvironment demonstrated a trend toward reduced regulatory T cells during the first 3 months post-transplant followed by an increase in NK cells and monocytes in patients achieving a complete remission.
Conclusions: This phase 1 clinical trial demonstrates that early introduction of immunotherapy after ASCT is well tolerated and shows promising disease control in patients with MM, accompanied by favorable changes in the immune microenvironment.
Trial registration number: NCT02655458.
Keywords: hematologic neoplasms; immunotherapy; transplantation immunology; tumor microenvironment.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DGC, KO, AA, SK-S, SB, and SK declare no conflict of interest. DC reports participation in the speakers’ bureau for Bristol Myers Squibb (BMS). SP reports consulting fees from Foundation Medicine and research funding from BMS (Celgene), Karyopharm, and Amgen. SG reports current and past research funding from Regeneron Pharmaceuticals, Boehringer Ingelheim, BMS (Celgene), Genentech, EMD Serono, Pfizer, and Takeda, unrelated to the current work. SJ reports consulting fees from Janssen, BMS, Regeneron, Sanofi, Takeda, and Karyopharm and data monitoring committee participation for Genmab and Sanofi. HJC reports employment by the Multiple Myeloma Research Foundation and receives research funding from BMS and Takeda, Inc, for work unrelated to this study.
Figures


References
-
- Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. American J Hematol 2022;97:1086–107. 10.1002/ajh.26590 Available: https://onlinelibrary.wiley.com/toc/10968652/97/8 - DOI - PMC - PubMed
-
- Landgren O, Hultcrantz M, Diamond B, et al. . Safety and effectiveness of weekly Carfilzomib, Lenalidomide, dexamethasone, and Daratumumab combination therapy for patients with newly diagnosed multiple myeloma: the MANHATTAN Nonrandomized clinical trial. JAMA Oncol 2021;7:862–8. 10.1001/jamaoncol.2021.0611 - DOI - PMC - PubMed
-
- Moreau P, Attal M, Hulin C, et al. . Bortezomib, thalidomide, and dexamethasone with or without Daratumumab before and after Autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 2019;394:29–38. 10.1016/S0140-6736(19)31240-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
