Armored TGFβRIIDN ROR1-CAR T cells reject solid tumors and resist suppression by constitutively-expressed and treatment-induced TGFβ1
- PMID: 38609317
- PMCID: PMC11029479
- DOI: 10.1136/jitc-2023-008261
Armored TGFβRIIDN ROR1-CAR T cells reject solid tumors and resist suppression by constitutively-expressed and treatment-induced TGFβ1
Abstract
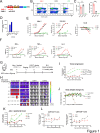
Background: Chimeric antigen receptor (CAR) T-cell therapy target receptor tyrosine kinase-like orphan receptor 1 (ROR1) is broadly expressed in hematologic and solid tumors, however clinically-characterized ROR1-CAR T cells with single chain variable fragment (scFv)-R12 targeting domain failed to induce durable remissions, in part due to the immunosuppressive tumor microenvironment (TME). Herein, we describe the development of an improved ROR1-CAR with a novel, fully human scFv9 targeting domain, and augmented with TGFβRIIDN armor protective against a major TME factor, transforming growth factor beta (TGFβ).
Methods: CAR T cells were generated by lentiviral transduction of enriched CD4+ and CD8+ T cells, and the novel scFv9-based ROR1-CAR-1 was compared with the clinically-characterized ROR1-R12-scFv-based CAR-2 in vitro and in vivo.
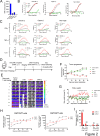
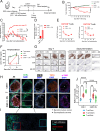
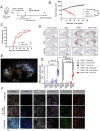
Results: CAR-1 T cells exhibited greater CAR surface density than CAR-2 when normalized for %CAR+, and produced more interferon (IFN)-γ tumor necrosis factor (TNF)-α and interleukin (IL)-2 in response to hematologic (Jeko-1, RPMI-8226) and solid (OVCAR-3, Capan-2, NCI-H226) tumor cell lines in vitro. In vivo, CAR-1 and CAR-2 both cleared hematologic Jeko-1 lymphoma xenografts, however only CAR-1 fully rejected ovarian solid OVCAR-3 tumors, concordantly with greater expansion of CD8+ and CD4+CAR T cells, and enrichment for central and effector memory phenotype. When equipped with TGFβ-protective armor TGFβRIIDN, CAR-1 T cells resisted TGFβ-mediated pSmad2/3 phosphorylation, as compared with CAR-1 alone. When co-cultured with ROR-1+ AsPC-1 pancreatic cancer line in the presence of TGFβ1, armored CAR-1 demonstrated improved recovery of killing function, IFN-γ, TNF-α and IL-2 secretion. In mouse AsPC-1 pancreatic tumor xenografts overexpressing TGFβ1, armored CAR-1, in contrast to CAR-1 alone, achieved complete tumor remissions, and yielded accelerated expansion of CAR+ T cells, diminished circulating active TGFβ1, and no apparent toxicity or weight loss. Unexpectedly, in AsPC-1 xenografts without TGFβ overexpression, TGFβ1 production was specifically induced by ROR-1-CAR T cells interaction with ROR-1 positive tumor cells, and the TGFβRIIDN armor conferred accelerated tumor clearance.
Conclusions: The novel fully human TGFßRIIDN-armored ROR1-CAR-1 T cells are highly potent against ROR1-positive tumors, and withstand the inhibitory effects of TGFß in solid TME. Moreover, TGFβ1 induction represents a novel, CAR-induced checkpoint in the solid TME, which can be circumvented by co-expressing the TGβRIIDN armor on T cells.
Keywords: Cell Engineering; Immunotherapy, Adoptive; Receptors, Chimeric Antigen; T-Lymphocytes; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors are employees of Lentigen Technology Inc., a Miltenyi Biotec Company.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
