Novel collector design and optimized photo-fenton model for sustainable industry textile wastewater treatment
- PMID: 38609385
- PMCID: PMC11014862
- DOI: 10.1038/s41598-024-58610-w
Novel collector design and optimized photo-fenton model for sustainable industry textile wastewater treatment
Abstract
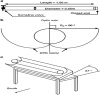
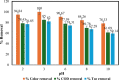
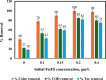
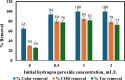
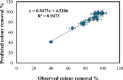
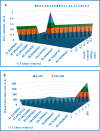
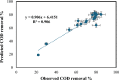
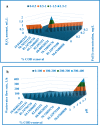
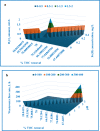
Textile industry wastewater containing toxic dyes and high COD poses environmental hazards and requires treatment before discharge. This study addresses the challenge of treating complex textile wastewater using a novel integrated system. The system combines sedimentation, screening, adsorption, and an optimized solar photo-Fenton process to provide a sustainable treatment solution. A novel parabolic collector with a larger absorber tube diameter enhances solar radiation utilization at lower catalyst concentrations. This design is versatile, treating all types of wastewaters, especially those that contain colors, smells, solid and suspended materials, in addition to its importance for the treatment of difficult substances that may be present in industrial and sewage wastewaters that are difficult to dispose of by traditional treatment methods. Multivariate experiments optimized key photo-Fenton parameters (pH, catalyst dose, etc.) achieving significant pollutant removal (85% COD, 82% TOC, complete color) under specific conditions (pH 3, 0.2 g/L Fe(II), 1 mL/L H2O2, 40 °C and 100 L/h flow rate after 60 min irradiation). Kinetic modeling revealed second-order reaction kinetics, and multivariate regression analysis led to the development of models predicting treatment efficiency based on process factors. The key scientific contributions are the integrated system design combining conventional and advanced oxidation technologies, novel collector configuration for efficient utilization of solar radiation, comprehensive process optimization through multivariate experiments, kinetic modeling and predictive modeling relating process factors to pollutant degradation. This provides an economical green solution for textile wastewater treatment and reuse along with useful design guidelines. The treatment methodology and modeling approach make valuable additions for sustainable management of textile industry wastewater.
Keywords: Classical processes; Developed oxidation processes; Free hydroxyl radicals; Integrated unit; Solar photo-fenton process; Wastewater treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Similar articles
-
Application of solar photo-Fenton toward toxicity removal and textile wastewater reuse.Environ Sci Pollut Res Int. 2017 May;24(14):12515-12528. doi: 10.1007/s11356-016-7395-5. Epub 2016 Aug 27. Environ Sci Pollut Res Int. 2017. PMID: 27566160
-
Enhancing textile wastewater reuse: Integrating Fenton oxidation with membrane filtration.J Environ Manage. 2025 Apr;379:124873. doi: 10.1016/j.jenvman.2025.124873. Epub 2025 Mar 7. J Environ Manage. 2025. PMID: 40056596
-
Comparison of kinetics and costs of Fenton and photo-Fenton processes used for the treatment of a textile industry wastewater.J Environ Manage. 2022 Feb 15;304:114234. doi: 10.1016/j.jenvman.2021.114234. Epub 2021 Dec 6. J Environ Manage. 2022. PMID: 34883439
-
Advanced oxidation process for the treatment of industrial wastewater: A review on strategies, mechanisms, bottlenecks and prospects.Chemosphere. 2023 Dec;345:140473. doi: 10.1016/j.chemosphere.2023.140473. Epub 2023 Oct 20. Chemosphere. 2023. PMID: 37866496 Review.
-
A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies.Chemosphere. 2020 Jul;250:126198. doi: 10.1016/j.chemosphere.2020.126198. Epub 2020 Feb 17. Chemosphere. 2020. PMID: 32105855 Review.
References
-
- Arslan I, AkmehmetBalcioglu I, Tuhkanen T. Advanced treatment of dyehouse effluents by Fe(II) and Mn(II)-catalyzed ozonation and the H2O2/O3 process. Water Sci. Technol. 2000;42:13–18.
-
- Sevimli MF, Kinaci C. Decolorization of textile wastewater by ozonation and Fenton’s process. Water Sci. Technol. 2002;45:279–286. - PubMed
-
- InanBeydilli M, Pavlostathis SG. Decolorization kinetics of the azo dye Reactive Red 2 under methanogenic conditions: effect of long-term culture acclimation. Biodegradation. 2005;16:135–146. - PubMed
-
- Moreira MT, Mielgo I, Feijoo G, Lema JM. Evaluation of different fungal strains in decolorization of synthetic dyes. Biotechnol. Lett. 2000;22:1499–1503.
-
- Arslan-Alaton I. A review of the effects of dye-assisting chemicals on advanced oxidation of reactive dyes in wastewater. Color. Technol. 2003;119:345–353.
LinkOut - more resources
Full Text Sources

