Rarefied gas flow in functionalized microchannels
- PMID: 38609441
- PMCID: PMC11014946
- DOI: 10.1038/s41598-024-59027-1
Rarefied gas flow in functionalized microchannels
Abstract
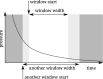
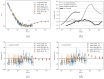
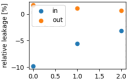
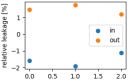
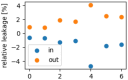
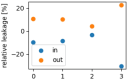
The interaction of rarefied gases with functionalized surfaces is of great importance in technical applications such as gas separation membranes and catalysis. To investigate the influence of functionalization and rarefaction on gas flow rate in a defined geometry, pressure-driven gas flow experiments with helium and carbon dioxide through plain and alkyl-functionalized microchannels are performed. The experiments cover Knudsen numbers from 0.01 to 200 and therefore the slip flow regime up to free molecular flow. To minimize the experimental uncertainty which is prevalent in micro flow experiments, a methodology is developed to make optimal use of the measurement data. The results are compared to an analysis-based hydraulic closure model (ACM) predicting rarefied gas flow in straight channels and to numerical solutions of the linearized S-model and BGK kinetic equations. The experimental data shows that if there is a difference between plain and functionalized channels, it is likely obscured by experimental uncertainty. This stands in contrast to previous measurements in smaller geometries and demonstrates that the surface-to-volume ratio of 0.4 m seems to be too small for the functionalization to have a strong influence and highlights the importance of geometric scale for surface effects. These results also shed light on the molecular reflection characteristics described by the TMAC.
Keywords: BGK equations; Carbon dioxide; Gas separation membranes; Knudsen number; Mesopores; S-model; TMAC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Baker, R. W. Membrane Technology and Applications. 10.1002/9781118359686.ch2 (Wiley , 2012).
-
- Montessori A, Prestininzi P, La Rocca M, et al. Effects of Knudsen diffusivity on the effective reactivity of nanoporous catalyst media. J. Comput. Sci. 2016;17:377–383. doi: 10.1016/j.jocs.2016.04.006. - DOI
-
- Jousten K. On the gas species dependence of Pirani vacuum gauges. J. Vac. Sci. Technol. A Vac. Surf. Films. 2008;26(3):352–359. doi: 10.1116/1.2897314. - DOI
-
- White C, Colombo C, Scanlon TJ, et al. Rarefied gas effects on the aerodynamics of high area-to-mass ratio spacecraft in orbit. Adv. Sp. Res. 2013;51(11):2112–2124. doi: 10.1016/j.asr.2013.01.002. - DOI
-
- Shen C. Rarefied Gas Dynamics—Fundamentals. Simulations and Micro Flows. Springer; 2005.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

