Protein restriction during pregnancy alters Cdkn1c silencing, dopamine circuitry and offspring behaviour without changing expression of key neuronal marker genes
- PMID: 38609446
- PMCID: PMC11014953
- DOI: 10.1038/s41598-024-59083-7
Protein restriction during pregnancy alters Cdkn1c silencing, dopamine circuitry and offspring behaviour without changing expression of key neuronal marker genes
Abstract
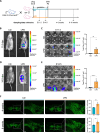
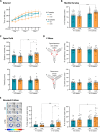
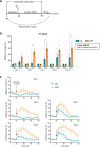
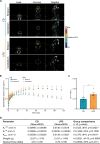
We tracked the consequences of in utero protein restriction in mice throughout their development and life course using a luciferase-based allelic reporter of imprinted Cdkn1c. Exposure to gestational low-protein diet (LPD) results in the inappropriate expression of paternally inherited Cdkn1c in the brains of embryonic and juvenile mice. These animals were characterised by a developmental delay in motor skills, and by behavioural alterations indicative of reduced anxiety. Exposure to LPD in utero resulted in significantly more tyrosine hydroxylase positive (dopaminergic) neurons in the midbrain of adult offspring as compared to age-matched, control-diet equivalents. Positron emission tomography (PET) imaging revealed an increase in striatal dopamine synthesis capacity in LPD-exposed offspring, where elevated levels of dopamine correlated with an enhanced sensitivity to cocaine. These data highlight a profound sensitivity of the developing epigenome to gestational protein restriction. Our data also suggest that loss of Cdkn1c imprinting and p57KIP2 upregulation alters the cellular composition of the developing midbrain, compromises dopamine circuitry, and thereby provokes behavioural abnormalities in early postnatal life. Molecular analyses showed that despite this phenotype, exposure to LPD solely during pregnancy did not significantly change the expression of key neuronal- or dopamine-associated marker genes in adult offspring.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

