Deciphering antifungal and antibiofilm mechanisms of isobavachalcone against Cryptococcus neoformans through RNA-seq and functional analyses
- PMID: 38609931
- PMCID: PMC11015616
- DOI: 10.1186/s12934-024-02369-2
Deciphering antifungal and antibiofilm mechanisms of isobavachalcone against Cryptococcus neoformans through RNA-seq and functional analyses
Abstract
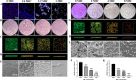
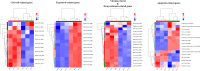
Cryptococcus neoformans has been designated as critical fungal pathogens by the World Health Organization, mainly due to limited treatment options and the prevalence of antifungal resistance. Consequently, the utilization of novel antifungal agents is crucial for the effective treatment of C. neoformans infections. This study exposed that the minimum inhibitory concentration (MIC) of isobavachalcone (IBC) against C. neoformans H99 was 8 µg/mL, and IBC dispersed 48-h mature biofilms by affecting cell viability at 16 µg/mL. The antifungal efficacy of IBC was further validated through microscopic observations using specific dyes and in vitro assays, which confirmed the disruption of cell wall/membrane integrity. RNA-Seq analysis was employed to decipher the effect of IBC on the C. neoformans H99 transcriptomic profiles. Real-time quantitative reverse transcription PCR (RT-qPCR) analysis was performed to validate the transcriptomic data and identify the differentially expressed genes. The results showed that IBC exhibited various mechanisms to impede the growth, biofilm formation, and virulence of C. neoformans H99 by modulating multiple dysregulated pathways related to cell wall/membrane, drug resistance, apoptosis, and mitochondrial homeostasis. The transcriptomic findings were corroborated by the antioxidant analyses, antifungal drug sensitivity, molecular docking, capsule, and melanin assays. In vivo antifungal activity analysis demonstrated that IBC extended the lifespan of C. neoformans-infected Caenorhabditis elegans. Overall, the current study unveiled that IBC targeted multiple pathways simultaneously to inhibit growth significantly, biofilm formation, and virulence, as well as to disperse mature biofilms of C. neoformans H99 and induce cell death.
Keywords: Cryptococcus neoformans; Antifungal activity; Functional profiling; Isobavachalcone; RNA-sequencing.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was performed in the absence of any commercial or financial relationships that can be considered as a potential conflict of interest.
Figures







References
MeSH terms
Substances
Grants and funding
- L2023-ZDYF-QYCX-059/the science and technology plan project of Xianyang science and technology bureau
- 2023-YBNY-170/Key Research and Development Plan Project in Shaanxi Province.
- NYKJ-2023-(XA)02/Shaanxi province agricultural science and technology innovation project
- 81973531/Natural science foundation of China
- 20231120113324002/The fundamental research project of the Shenzhen Science and Technology Innovation Commission
LinkOut - more resources
Full Text Sources

